The use of equine chondrogenic-induced mesenchymal stem cells as a treatment for osteoarthritis: A randomised, double-blinded, placebo-controlled proof-of-concept study
- PMID: 30815897
- PMCID: PMC6850029
- DOI: 10.1111/evj.13089
The use of equine chondrogenic-induced mesenchymal stem cells as a treatment for osteoarthritis: A randomised, double-blinded, placebo-controlled proof-of-concept study
Abstract
Background: There is a need to improve therapies for osteoarthritis in horses.
Objectives: To assess the efficacy of equine allogeneic chondrogenic-induced mesenchymal stem cells combined with equine allogeneic plasma as a novel therapy for osteoarthritis in horses.
Study design: Randomised, double-blinded, placebo-controlled experiment.
Methods: In 12 healthy horses, osteoarthritis was induced in the metacarpophalangeal joint using an osteochondral fragment-groove model. Five weeks after surgery, horses were randomly assigned to either an intra-articular injection with chondrogenic-induced mesenchymal stem cells + equine allogeneic plasma (= intervention) or with 0.9% saline solution (= control). From surgery until the study end, horses underwent a weekly joint and lameness assessment. Synovial fluid was collected for cytology and biomarker analysis before surgery and at Weeks 5, 5 + 1d, 7, 9 and 11. At Week 11, horses were subjected to euthanasia, and the metacarpophalangeal joints were evaluated macroscopically and histologically.
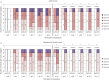
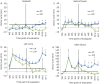
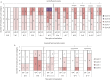
Results: No serious adverse events or suspected adverse drug reactions occurred during the study. A significant improvement in visual and objective lameness was seen with the intervention compared with the control. Synovial fluid displayed a significantly higher viscosity and a significantly lower glycosaminoglycan concentration in the intervention group. Other biomarkers or cytology parameters were not significantly different between the treatment groups. Significantly less wear lines and synovial hyperaemia were present in the intervention group. The amount of cartilage oligomeric matrix protein, collagen type II and glycosaminoglycans were significantly higher in the articular cartilage of the intervention group.
Main limitations: This study assessed the short-term effect of the intervention on a limited number of horses, using an osteoarthritis model. This study also included multiple statistical tests, increasing the risk of type 1 error.
Conclusions: Equine allogeneic chondrogenic-induced mesenchymal stem cells combined with equine allogeneic plasma may be a promising treatment for osteoarthritis in horses. The Summary is available in Spanish - see Supporting Information.
Keywords: allogeneic; horse; metacarpophalangeal joint; model; peripheral blood.
© 2019 Global International Stem cell Technology (GST) NV. Equine Veterinary Journal published by John Wiley & Sons Ltd on behalf of EVJ Ltd.
Figures


References
-
- Goodrich, L.R. and Nixon, A.J. (2006) Medical treatment of osteoarthritis in the horse ‐ a review. Vet. J. 171, 51‐69. - PubMed
-
- Ferris, D.J. , Frisbie, D.D. , McIlwraith, C.W. and Kawcak, C.E. (2011) Current joint therapy usage in equine practice: a survey of veterinarians 2009. Equine Vet. J. 43, 530‐535. - PubMed
-
- Frisbie, D.D. , Kisiday, J.D. , Kawcak, C.E. , Werpy, N.M. and McIlwraith, C.W. (2009) Evaluation of adipose‐derived stromal vascular fraction or bone marrow‐derived mesenchymal stem cells for treatment of osteoarthritis. J. Orthop. Res. 27, 1675‐1680. - PubMed
-
- Wilke, M.M. , Nydam, D.V. and Nixon, A.J. (2007) Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. J. Orthop. Res. 25, 913‐925. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

