Short-term starvation stress at young adult stages enhances meiotic activity of germ cells to maintain spermatogenesis in aged male Caenorhabditis elegans
- PMID: 30816005
- PMCID: PMC6516166
- DOI: 10.1111/acel.12930
Short-term starvation stress at young adult stages enhances meiotic activity of germ cells to maintain spermatogenesis in aged male Caenorhabditis elegans
Abstract
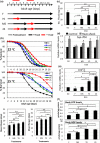
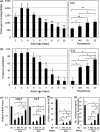
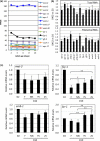
To survive and reproduce, living organisms must evolve numerous mechanisms to re-adjust their physiology when encountering adverse conditions that subject them to severe stress. We found that short-term starvation (STS) stress in young adult male Caenorhabditis elegans can significantly improve their vitality (relative to nonstressed males) when they are aged. In addition, we found that stress-treated aged males maintained reproductive activity equivalent to young males, whereas nonstressed aged males quickly lost reproductive ability. STS stress can preserve sperm number and quality in aged male worms. Spermatogenesis involves germ cell mitosis and meiosis. We found that germ cell meiotic activity is more sensitive to aging than mitotic activity and is declining rapidly with age. We examined the role of numerous factors important for spermatogenesis on STS-preserved spermatogenesis during aging. Our results show that mutant strains deficient in anaphase-promoting complex/cyclosome (APC/C) function fail to exhibit the STS stress-enhanced spermatogenesis found in wild-type N2 worms, suggesting that the mechanism underlying starvation-induced spermatogenesis involves the APC/C complex, a conserved ubiquitin-protein ligase E3 complex. Furthermore, transgenic expression of FZY-1/CDC-20, a coactivator of APC/C, ameliorated the age-associated decline of meiosis, similar to the hormetic effect of STS.
Keywords: CDC-20; anaphase-promoting complex/cyclosome (APC/C); meiosis; short-term starvation; spermatogenesis; stress response hormesis.
2019 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

