Thermally and Magnetically Robust Triplet Ground State Diradical
- PMID: 30816035
- PMCID: PMC6472266
- DOI: 10.1021/jacs.9b00558
Thermally and Magnetically Robust Triplet Ground State Diradical
Abstract
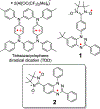
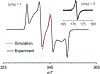
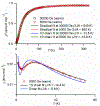
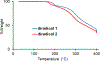
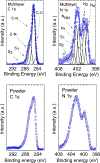
High spin ( S = 1) organic diradicals may offer enhanced properties with respect to several emerging technologies, but typically exhibit low singlet triplet energy gaps and possess limited thermal stability. We report triplet ground state diradical 2 with a large singlet-triplet energy gap, Δ EST ≥ 1.7 kcal mol-1, leading to nearly exclusive population of triplet ground state at room temperature, and good thermal stability with onset of decomposition at ∼160 °C under inert atmosphere. Magnetic properties of 2 and the previously prepared diradical 1 are characterized by SQUID magnetometry of polycrystalline powders, in polystyrene glass, and in other matrices. Polycrystalline diradical 2 forms a novel one-dimensional (1D) spin-1 ( S = 1) chain of organic radicals with intrachain antiferromagnetic coupling of J'/ k = -14 K, which is associated with the N···N and N···O intermolecular contacts. The intrachain antiferromagnetic coupling in 2 is by far strongest among all studied 1D S = 1 chains of organic radicals, which also makes 1D S = 1 chains of 2 most isotropic, and therefore an excellent system for studies of low-dimensional magnetism. In polystyrene glass and in frozen benzene or dibutyl phthalate solution, both 1 and 2 are monomeric. Diradical 2 is thermally robust and is evaporated under ultrahigh vacuum to form thin films of intact diradicals on silicon substrate, as demonstrated by X-ray photoelectron spectroscopy. Based on C-K NEXAFS spectra and AFM images of the ∼1.5 nm thick films, the diradical molecules form islands on the substrate with molecules stacked approximately along the crystallographic a-axis. The films are stable under ultrahigh vacuum for at least 60 h but show signs of decomposition when exposed to ambient conditions for 7 h.
Figures




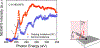
References
-
- Rajca A; Wongsriratanakul J; Rajca S Magnetic ordering in an organic polymer. Science 2001, 294, 1503–1505. - PubMed
-
- Ratera L; Veciana J Playing with organic radicals as building blocks for functional molecular materials. Chem. Soc. Rev. 2012, 41, 303–349. - PubMed
-
- Wingate AJ; Boudouris BW Recent advances in the syntheses of radical-containing macromolecules. J. Polym. Sci., Part A: Polym. Chem. 2016, 54, 1875–1894.
-
- Rajca A Organic diradicals and polyradicals: from spin coupling to magnetism?. Chem. Rev. 1994, 94, 871–893.
- (b) Rajca A The Physical Organic Chemistry of Very High-Spin Polyradicals. Adv. Phys. Org. Chem. 2005, 40, 153–199.
- (c) Gallagher NM; Arnon Olankitwanit A; Rajca A High-Spin Organic Molecules. J. Org. Chem. 2015, 80, 1291–1298. - PubMed
-
- Rajca A; Lu K; Rajca S High-spin polyarylmethyl polyradical: Fragment of a macrocyclic 2-strand based upon calix[4]arene rings. J. Am. Chem. Soc. 1997, 119, 10335–10345.
- (b) Rajca S; Rajca A; Wongsriratanakul J; Butler P; Choi S Organic Spin Clusters. Dendritic-Macrocyclic Polyarylmethyl Polyradical with Very High-Spin of S = 10 and its Derivatives: Synthesis, Magnetic Studies, and Small Angle Neutron Scattering. J. Am. Chem. Soc. 2004, 126, 6972–6986. - PubMed
- (c) Rajca A; Wongsriratanakul J; Rajca S; Cerny RL Organic Spin Clusters: Annelated Macrocyclic Polyarylmethyl Polyradicals and Polymer with Very High-Spin S = 6 – 18. Chem. Eur. J. 2004, 10, 3144–3157. - PubMed
- (d) Rajca A; Wongsriratanakul J; Rajca S Organic Spin Clusters: Macrocyclic-Macrocyclic Polyarylmethyl Polyradicals with Very High-Spin S = 5 – 13. J. Am. Chem. Soc. 2004, 126, 6608–6626. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

