Phase II trial of VEGFR2 inhibitor apatinib for metastatic sarcoma: focus on efficacy and safety
- PMID: 30816108
- PMCID: PMC6395676
- DOI: 10.1038/s12276-019-0221-7
Phase II trial of VEGFR2 inhibitor apatinib for metastatic sarcoma: focus on efficacy and safety
Abstract
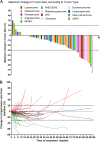
Apatinib (YN968D1) is a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor receptor 2 (VEGFR-2). We conducted a single-arm, nonrandomized phase II study (NCT03121846) to assess the efficacy and safety of apatinib in patients with stage IV sarcoma. We recruited 64 patients with stage IV sarcoma who had failed chemotherapy. The primary endpoint was progression-free survival (PFS), and the secondary endpoints were progression-free survival rate (PFR), objective response rate (ORR), and disease control rate (DCR) at week 12. Treatment-related adverse effects (AEs) were evaluated. Fifty-nine patients were assessed for efficacy and 64 patients for AEs. The median PFS was 7.93 months. At 12 weeks, the PFR was 74%, the ORR was 16.95% (10/59), and the DCR was 86.44% (51/59). The final ORR was 15.25% (9/59) and the DCR was 57.63% (34/59). Notably, 22 patients (34.38%) who developed hypertension, hand-foot-skin reaction, or proteinuria had significantly longer OS than those without these AEs (18.20 vs. 10.73 months; P = 0.002). We conclude that apatinib is effective and well tolerated in patients with advanced sarcoma. The development of hypertension, hand-foot-skin reaction, or proteinuria may indicate a favorable prognosis, representing a novel finding in sarcoma patients.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Efficacy and safety of the VEGFR2 inhibitor Apatinib for metastatic soft tissue sarcoma: Chinese cohort data from NCT03121846.Biomed Pharmacother. 2020 Feb;122:109587. doi: 10.1016/j.biopha.2019.109587. Epub 2019 Nov 28. Biomed Pharmacother. 2020. PMID: 31786466 Clinical Trial.
-
Clinical study of apatinib in the treatment of stage IV osteogenic sarcoma after failure of chemotherapy.Cancer Biol Med. 2020 May 15;17(2):501-512. doi: 10.20892/j.issn.2095-3941.2019.0397. Cancer Biol Med. 2020. PMID: 32587785 Free PMC article. Clinical Trial.
-
Efficacy and safety of apatinib in patients with advanced nonsmall cell lung cancer that failed prior chemotherapy or EGFR-TKIs: A pooled analysis.Medicine (Baltimore). 2018 Aug;97(35):e12083. doi: 10.1097/MD.0000000000012083. Medicine (Baltimore). 2018. PMID: 30170427 Free PMC article.
-
The safety of apatinib for the treatment of gastric cancer.Expert Opin Drug Saf. 2018 Nov;17(11):1145-1150. doi: 10.1080/14740338.2018.1535592. Epub 2018 Oct 24. Expert Opin Drug Saf. 2018. PMID: 30324820 Review.
-
Apatinib in recurrent anaplastic meningioma: a retrospective case series and systematic literature review.Cancer Biol Ther. 2020 Jul 2;21(7):583-589. doi: 10.1080/15384047.2020.1740053. Epub 2020 Mar 25. Cancer Biol Ther. 2020. PMID: 32212907 Free PMC article.
Cited by
-
VEGFR Inhibitors for Uterine Metastatic Perivascular Epithelioid Tumors (PEComa) Resistant to mTOR Inhibitors. A Case Report and Review of Literature.Front Oncol. 2021 Mar 26;11:641376. doi: 10.3389/fonc.2021.641376. eCollection 2021. Front Oncol. 2021. PMID: 33842348 Free PMC article. Review.
-
Clinical outcomes and safety of apatinib monotherapy in the treatment of patients with advanced epithelial ovarian carcinoma who progressed after standard regimens and the analysis of the VEGFR2 polymorphism.Oncol Lett. 2020 Sep;20(3):3035-3045. doi: 10.3892/ol.2020.11857. Epub 2020 Jul 10. Oncol Lett. 2020. PMID: 32782621 Free PMC article.
-
The role of apatinib combined with paclitaxel (aluminum binding type) in platinum-resistant ovarian cancer.J Ovarian Res. 2020 Sep 21;13(1):113. doi: 10.1186/s13048-020-00719-3. J Ovarian Res. 2020. Retraction in: J Ovarian Res. 2021 Feb 19;14(1):37. doi: 10.1186/s13048-021-00785-1. PMID: 32958014 Free PMC article. Retracted.
-
Management of Regorafenib-Induced Hand-Foot Skin Reaction with Topical Chinese Medicine and Urea Ointment: A Case Report and Literature Review.Onco Targets Ther. 2025 Apr 9;18:509-519. doi: 10.2147/OTT.S510766. eCollection 2025. Onco Targets Ther. 2025. PMID: 40225901 Free PMC article.
-
Anlotinib as a molecular targeted therapy for tumors.Oncol Lett. 2020 Aug;20(2):1001-1014. doi: 10.3892/ol.2020.11685. Epub 2020 May 28. Oncol Lett. 2020. PMID: 32724339 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

