Hyper-acidic fusion minipeptides escort the intrinsic antioxidative ability of the pattern recognition receptor CRP in non-animal organisms
- PMID: 30816172
- PMCID: PMC6395739
- DOI: 10.1038/s41598-019-39388-8
Hyper-acidic fusion minipeptides escort the intrinsic antioxidative ability of the pattern recognition receptor CRP in non-animal organisms
Abstract
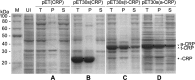
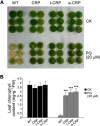
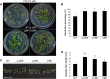
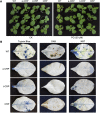
C-reactive protein (CRP) is widely used as a biomarker of inflammation. It plays important roles in innate immunity response as a member of pattern recognition receptors, by binding oxidation-specific epitopes including some intermediates of lipid oxidative chain reaction. The inferred antioxidative ability of CRP was ever demonstrated by only few in vitro evidences, and needs to be clarified especially in vivo. Herein, we expressed human CRP in three representative non-animal organisms (Escherichia coli, Saccharomyces cerevisiae, and tobacco) inherently lacking the milieu for CRP signalling, and found CRP did possess an intrinsic antioxidative ability. Heterologous CRP could confer increased oxidative resistance in its recombinant E. coli and yeast cells and transgenic tobaccos. We also revealed a positive correlation between the antioxidative effect of CRP and its solubility. Only soluble CRP could exhibit distinct antioxidative activity, while the CRP aggregates might be instead toxic (probably pro-oxidative) to cells. Moreover, fusion with hyper-acidic minipeptides could remarkably improve CRP solubility, and meanwhile guarantee or enhance CRP antioxidative ability. These results not only provide a new insight for understanding the etiology of CRP-involved inflammations and diseases, and also endorse a potential of CRP biotechnological applications in developing new pharmaceutical therapies and improving plant oxidative resistance.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Potentiation of the activity of Escherichia coli chaperone DnaJ by tailing hyper-acidic minipeptides.J Biotechnol. 2021 Nov 20;341:86-95. doi: 10.1016/j.jbiotec.2021.09.012. Epub 2021 Sep 24. J Biotechnol. 2021. PMID: 34563565
-
Hyper-acidic protein fusion partners improve solubility and assist correct folding of recombinant proteins expressed in Escherichia coli.J Biotechnol. 2008 Jul 31;135(4):333-9. doi: 10.1016/j.jbiotec.2008.05.007. Epub 2008 May 27. J Biotechnol. 2008. PMID: 18599143
-
Human SUMO fusion systems enhance protein expression and solubility.Protein Expr Purif. 2010 Oct;73(2):203-8. doi: 10.1016/j.pep.2010.05.001. Epub 2010 May 10. Protein Expr Purif. 2010. PMID: 20457256
-
C-Reactive Protein and Cancer: Interpreting the Differential Bioactivities of Its Pentameric and Monomeric, Modified Isoforms.Front Immunol. 2021 Sep 6;12:744129. doi: 10.3389/fimmu.2021.744129. eCollection 2021. Front Immunol. 2021. PMID: 34552600 Free PMC article. Review.
-
Function of C-reactive protein.Ann Med. 2000 May;32(4):274-8. doi: 10.3109/07853890009011772. Ann Med. 2000. PMID: 10852144 Review.
Cited by
-
Response of Prolyl 4 Hydroxylases, Arabinogalactan Proteins and Homogalacturonans in Four Olive Cultivars under Long-Term Salinity Stress in Relation to Physiological and Morphological Changes.Cells. 2023 May 24;12(11):1466. doi: 10.3390/cells12111466. Cells. 2023. PMID: 37296587 Free PMC article.
-
Human Fcγ-receptors selectively respond to C-reactive protein isoforms.Front Immunol. 2025 May 19;16:1598605. doi: 10.3389/fimmu.2025.1598605. eCollection 2025. Front Immunol. 2025. PMID: 40458392 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

