Elucidating the Contribution of Skeletal Muscle Ion Channels to Amyotrophic Lateral Sclerosis in search of new therapeutic options
- PMID: 30816241
- PMCID: PMC6395744
- DOI: 10.1038/s41598-019-39676-3
Elucidating the Contribution of Skeletal Muscle Ion Channels to Amyotrophic Lateral Sclerosis in search of new therapeutic options
Abstract
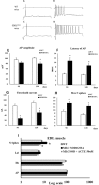
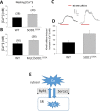
The discovery of pathogenetic mechanisms is essential to identify new therapeutic approaches in Amyotrophic Lateral Sclerosis (ALS). Here we investigated the role of the most important ion channels in skeletal muscle of an ALS animal model (MLC/SOD1G93A) carrying a mutated SOD1 exclusively in this tissue, avoiding motor-neuron involvement. Ion channels are fundamental proteins for muscle function, and also to sustain neuromuscular junction and nerve integrity. By a multivariate statistical analysis, using machine learning algorithms, we identified the discriminant genes in MLC/SOD1G93A mice. Surprisingly, the expression of ClC-1 chloride channel, present only in skeletal muscle, was reduced. Also, the expression of Protein Kinase-C, known to control ClC-1 activity, was increased, causing its inhibition. The functional characterization confirmed the reduction of ClC-1 activity, leading to hyperexcitability and impaired relaxation. The increased expression of ion channel coupled AMPA-receptor may contribute to sustained depolarization and functional impairment. Also, the decreased expression of irisin, a muscle-secreted peptide protecting brain function, may disturb muscle-nerve connection. Interestingly, the in-vitro application of chelerythrine or acetazolamide, restored ClC-1 activity and sarcolemma hyperexcitability in these mice. These findings show that ion channel function impairment in skeletal muscle may lead to motor-neuron increased vulnerability, and opens the possibility to investigate on new compounds as promising therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Progressive impairment of CaV1.1 function in the skeletal muscle of mice expressing a mutant type 1 Cu/Zn superoxide dismutase (G93A) linked to amyotrophic lateral sclerosis.Skelet Muscle. 2016 Jun 23;6:24. doi: 10.1186/s13395-016-0094-6. eCollection 2016. Skelet Muscle. 2016. PMID: 27340545 Free PMC article.
-
Granulocyte Colony-Stimulating Factor Ameliorates Skeletal Muscle Dysfunction in Amyotrophic Lateral Sclerosis Mice and Improves Proliferation of SOD1-G93A Myoblasts in vitro.Neurodegener Dis. 2017;17(1):1-13. doi: 10.1159/000446113. Epub 2016 Aug 20. Neurodegener Dis. 2017. PMID: 27544379
-
Macrophage-mediated inflammation and glial response in the skeletal muscle of a rat model of familial amyotrophic lateral sclerosis (ALS).Exp Neurol. 2016 Mar;277:275-282. doi: 10.1016/j.expneurol.2016.01.008. Epub 2016 Jan 13. Exp Neurol. 2016. PMID: 26775178 Free PMC article.
-
Skeletal Muscle Metabolism: Origin or Prognostic Factor for Amyotrophic Lateral Sclerosis (ALS) Development?Cells. 2021 Jun 9;10(6):1449. doi: 10.3390/cells10061449. Cells. 2021. PMID: 34207859 Free PMC article. Review.
-
Therapeutic Targets in Amyotrophic Lateral Sclerosis: Focus on Ion Channels and Skeletal Muscle.Cells. 2022 Jan 25;11(3):415. doi: 10.3390/cells11030415. Cells. 2022. PMID: 35159225 Free PMC article. Review.
Cited by
-
Unlocking the Therapeutic Potential of Irisin: Harnessing Its Function in Degenerative Disorders and Tissue Regeneration.Int J Mol Sci. 2023 Mar 31;24(7):6551. doi: 10.3390/ijms24076551. Int J Mol Sci. 2023. PMID: 37047523 Free PMC article. Review.
-
From Riluzole to Dexpramipexole via Substituted-Benzothiazole Derivatives for Amyotrophic Lateral Sclerosis Disease Treatment: Case Studies.Molecules. 2020 Jul 22;25(15):3320. doi: 10.3390/molecules25153320. Molecules. 2020. PMID: 32707914 Free PMC article. Review.
-
Statin-Induced Myopathy: Translational Studies from Preclinical to Clinical Evidence.Int J Mol Sci. 2021 Feb 19;22(4):2070. doi: 10.3390/ijms22042070. Int J Mol Sci. 2021. PMID: 33669797 Free PMC article. Review.
-
The 2-Oxoglutarate Carrier Is S-Nitrosylated in the Spinal Cord of G93A Mutant hSOD1 Mice Resulting in Disruption of Mitochondrial Glutathione Transport.Biomedicines. 2022 Dec 27;11(1):61. doi: 10.3390/biomedicines11010061. Biomedicines. 2022. PMID: 36672568 Free PMC article.
-
Dysregulation of AMPA receptor subunit expression in sporadic ALS post-mortem brain.J Pathol. 2020 Jan;250(1):67-78. doi: 10.1002/path.5351. Epub 2019 Nov 23. J Pathol. 2020. PMID: 31579943 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

