Identification of P2Y receptors involved in oleamide-suppressing inflammatory responses in murine microglia and human dendritic cells
- PMID: 30816271
- PMCID: PMC6395661
- DOI: 10.1038/s41598-019-40008-8
Identification of P2Y receptors involved in oleamide-suppressing inflammatory responses in murine microglia and human dendritic cells
Abstract
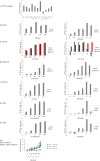
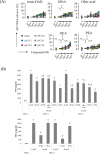
Microglia, a type of immune cell in the central nervous system, are involved in inflammation leading to neurodegenerative diseases. We previously identified oleamide from fermented dairy products as a neuroprotective compound suppressing microglial inflammation. Oleamide is an endocannabinoid and displays anti-inflammatory activity via the cannabinoid-2 (CB2) receptor; however, the mechanism underlying this anti-inflammatory activity has not been fully elucidated. Here, we found that the suppressive effect of oleamide on microglial tumor necrosis factor-α (TNF-α) production was canceled by inhibitors of G-protein-coupled receptor (GPCR) downstream signaling but not by a CB2 antagonist, suggesting that GPCRs other than CB2 are involved in the anti-inflammatory effects of oleamide. An extensive screen for GPCRs using a transforming growth factor-α shedding assay system identified P2Y1, P2Y4, P2Y6, P2Y10, and P2Y11 as candidates for the oleamide target. P2Y1 and P2Y10 agonists suppressed microglial TNF-α production, while a pan P2 receptor antagonist canceled the suppressive effect. Furthermore, we observed a relationship between the P2Y1 agonistic activities and the suppressive activities of oleamide and its analogs. Taken together, our results suggest that, in addition to CB2, P2Y type receptors are the potential targets of oleamide, and P2Y1 plays a role in the suppression of microglial inflammatory responses by oleamide. (200/200 words).
Conflict of interest statement
M.K. and Y.A. are employed by Kirin Co. Ltd. All other authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

