Cancer associated fibroblasts sculpt tumour microenvironment by recruiting monocytes and inducing immunosuppressive PD-1+ TAMs
- PMID: 30816272
- PMCID: PMC6395633
- DOI: 10.1038/s41598-019-39553-z
Cancer associated fibroblasts sculpt tumour microenvironment by recruiting monocytes and inducing immunosuppressive PD-1+ TAMs
Abstract
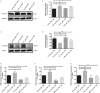
Fibroblasts turn into cancer associated fibroblasts (CAFs) in the tumour microenvironment. CAFs have recently attracted attention for their function as a regulator of immune cell recruitment and function in addition to their tumour-promoting roles. In this study, we aimed to determine the role of CAFs on monocyte recruitment and macrophage polarization in breast cancer. CAFs, which were α-SMA expressing fibroblasts in contrast to normal fibroblasts (NFs), effectively recruited monocytes. Recruitment of monocytes by CAFs might be mediated by monocyte chemotactic protein-1 (MCP-1) as well as stromal cell-derived factor-1 (SDF-1) cytokines. CAFs differentiated the recruited monocytes into M2-like macrophages which are capable of exerting their immunosuppressive roles via the PD-1 axis. CAF-educated monocytes exhibited strong immune suppression unlike NF-educated monocytes and enhanced the motility/invasion of breast cancer cells in addition to increasing the expressions of epithelial-mesenchymal transition (EMT)-related genes and vimentin protein in cancer cells. CAF-educated M1 macrophages displayed increased expression of M2 markers and production of anti-inflammatory cytokine IL-10 in contrast to decreased production of pro-inflammatory cytokine IL-12 compared with control M1 macrophages; suggesting that CAFs were also able to induce the trans-differentiation of M1 macrophages to M2 macrophages. We then investigated the relationship between the infiltration of CAFs and tumour associated macrophages (TAMs) using tissue samples obtained from breast cancer patients. High grade of CAFs significantly correlated with the number of TAMs in human breast cancer tissue samples. It was also associated with higher Ki-67 proliferation index, and higher tumour volume. This result is in line with our finding of increased breast cancer cell proliferation due to the effects of CAF-educated monocytes in vitro. Our results concluded that CAFs play pivotal roles in sculpturing the tumour microenvironment in breast cancer, and therapeutic strategies to reverse the CAF-mediated immunosuppressive microenvironment should be taken into consideration.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

