Sorafenib in Hepatopulmonary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial
- PMID: 30816637
- PMCID: PMC6910867
- DOI: 10.1002/lt.25438
Sorafenib in Hepatopulmonary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
The tyrosine kinase inhibitor sorafenib improves hepatopulmonary syndrome (HPS) in an experimental model. However, the efficacy and adverse effect profile in patients with HPS are unknown. We aimed to determine the effect of sorafenib on the alveolar-arterial oxygen gradient (AaPO2 ) at 3 months in patients with HPS. We performed a randomized, double-blind, placebo-controlled parallel trial of sorafenib in patients with HPS at 7 centers. A total of 28 patients with HPS were randomized to sorafenib 400 mg by mouth daily or a matching placebo in a 1:1 ratio. We found no statistically significant difference in the median change in AaPO2 from baseline to 12 weeks between the patients allocated to sorafenib (4.5 mm Hg; IQR, -3.8 to 7.0 mm Hg) and those allocated to placebo (-2.4 mm Hg; IQR, -4.8 to 8.2 mm Hg; P = 0.70). There was also no difference between the groups in terms of degree of intrapulmonary shunting by contrast echocardiography. Sorafenib significantly reduced circulating levels of angiogenic markers, including vascular endothelial growth factor receptors (P < 0.01) and TIE2-expressing M2 monocytes (P = 0.03), but it reduced the mental component scores of the Short Form 36 (P = 0.04), indicating a worse quality of life. In conclusion, sorafenib did not change the AaPO2 or other disease markers at 3 months in patients with HPS. Alternative antiangiogenic therapies or treatments targeting other pathways should be investigated.
Copyright © 2019 by the American Association for the Study of Liver Diseases.
Figures

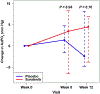
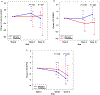
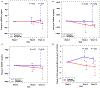
Comment in
-
Translational Research in Hepatopulmonary Syndrome: Lessons Learned Despite Negative Results.Liver Transpl. 2019 Aug;25(8):1136-1137. doi: 10.1002/lt.25586. Liver Transpl. 2019. PMID: 31206237 No abstract available.
References
-
- CDC/NCHS. National Vital Statistics System. Mortality. 2015.
-
- Swanson KL, Wiesner RH, Krowka MJ. Natural history of hepatopulmonary syndrome: impact of liver transplantation. Hepatology 2005;41:1122–1129. - PubMed
-
- Iyer VN, Swanson KL, Cartin-Ceba R, Dierkhising RA, Rosen CB, Heimbach JK, et al. Hepatopulmonary syndrome: favorable outcomes in the MELD exception era. Hepatology 2013;57:2427–2435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

