Denosumab Versus Risedronate in Glucocorticoid-Induced Osteoporosis: Final Results of a Twenty-Four-Month Randomized, Double-Blind, Double-Dummy Trial
- PMID: 30816640
- PMCID: PMC6619388
- DOI: 10.1002/art.40874
Denosumab Versus Risedronate in Glucocorticoid-Induced Osteoporosis: Final Results of a Twenty-Four-Month Randomized, Double-Blind, Double-Dummy Trial
Abstract
Objective: Clinical trial results have shown that, in glucocorticoid-treated patients, treatment with denosumab 60 mg subcutaneously once every 6 months (Q6M) increased spine and hip bone mineral density (BMD) at month 12 significantly more than treatment with risedronate 5 mg orally once daily (QD). The present analysis was performed to compare efficacy and characterize safety through month 24.
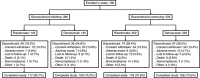
Methods: This phase III study enrolled men and women ≥18 years old who had received ≥7.5 mg daily prednisone or equivalent for <3 months (glucocorticoid-initiating) or for ≥3 months (glucocorticoid-continuing) before screening. All patients <50 years old had a history of osteoporotic fracture. Glucocorticoid-continuing patients ≥50 years old had T scores of -2.0 or less (or -1.0 or less with fracture history). Patients were randomized (1:1) to receive denosumab 60 mg subcutaneously Q6M or risedronate 5 mg orally QD for 24 months, with daily calcium and vitamin D.
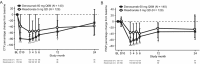
Results: Of 795 patients, 590 (74.2%) completed the study (in the glucocorticoid-initiating group, 109 of 145 patients treated with denosumab and 117 of 145 patients treated with risedronate; in the glucocorticoid-continuing group, 186 of 253 patients treated with denosumab and 178 of 252 patients treated with risedronate). Denosumab was superior to risedronate in increasing lumbar spine and total hip BMD at all time points assessed, among glucocorticoid-initiating patients (24-month lumbar spine: BMD increase of 6.2% versus 1.7%, respectively [P < 0.001]; 24-month total hip: BMD increase of 3.1% versus 0.0% [P < 0.001]) and among glucocorticoid-continuing patients (24-month lumbar spine: BMD increase of 6.4% versus 3.2% [P < 0.001]; 24-month total hip: BMD increase of 2.9% versus 0.5% [P < 0.001]). Adverse events, serious adverse events (including infections), and fractures were similar between treatment groups.
Conclusion: Denosumab was superior to risedronate in terms of increases in spine and hip BMD through month 24, and the safety profile was similar between treatment groups. Denosumab may offer a new osteoporosis treatment option for glucocorticoid-treated patients.
Trial registration: ClinicalTrials.gov NCT01575873.
© 2019 The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of American College of Rheumatology.
Figures

Comment in
-
Reply.Arthritis Rheumatol. 2019 Oct;71(10):1771-1772. doi: 10.1002/art.41013. Epub 2019 Sep 2. Arthritis Rheumatol. 2019. PMID: 31215773 No abstract available.
-
Bisphosphonates as a First-Line Treatment for Glucocorticoid-Induced Osteoporosis: Comment on the Article by Saag et al.Arthritis Rheumatol. 2019 Oct;71(10):1770-1771. doi: 10.1002/art.41016. Epub 2019 Aug 29. Arthritis Rheumatol. 2019. PMID: 31215777 No abstract available.
-
Where Does Denosumab Stand in the Treatment of Glucocorticoid-Induced Osteoporosis? Comment on the Article by Saag et al.Arthritis Rheumatol. 2019 Oct;71(10):1770. doi: 10.1002/art.41015. Epub 2019 Aug 29. Arthritis Rheumatol. 2019. PMID: 31216125 No abstract available.
References
-
- Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res (Hoboken) 2013;65:294–8. - PubMed
-
- Van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid‐induced osteoporosis: a meta‐analysis. Osteoporos Int 2002;13:777–87. - PubMed
-
- Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J, Hansen KE, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid‐induced osteoporosis. Arthritis Rheumatol 2017;69:1521–37. - PubMed
-
- Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, et al, for the Glucocorticoid‐Induced Osteoporosis Intervention Study Group . Alendronate for the prevention and treatment of glucocorticoid‐induced osteoporosis. N Engl J Med 1998;339:292–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

