Comparing Self-Monitoring Strategies for Weight Loss in a Smartphone App: Randomized Controlled Trial
- PMID: 30816851
- PMCID: PMC6416539
- DOI: 10.2196/12209
Comparing Self-Monitoring Strategies for Weight Loss in a Smartphone App: Randomized Controlled Trial
Abstract
Background: Self-monitoring of dietary intake is a valuable component of behavioral weight loss treatment; however, it declines quickly, thereby resulting in suboptimal treatment outcomes.
Objective: This study aimed to examine a novel behavioral weight loss intervention that aims to attenuate the decline in dietary self-monitoring engagement.
Methods: GoalTracker was an automated randomized controlled trial. Participants were adults with overweight or obesity (n=105; aged 21-65 years; body mass index, BMI, 25-45 kg/m2) and were randomized to a 12-week stand-alone weight loss intervention using the MyFitnessPal smartphone app for daily self-monitoring of either (1) both weight and diet, with weekly lessons, action plans, and feedback (Simultaneous); (2) weight through week 4, then added diet, with the same behavioral components (Sequential); or (3) only diet (App-Only). All groups received a goal to lose 5% of initial weight by 12 weeks, a tailored calorie goal, and automated in-app reminders. Participants were recruited via online and offline methods. Weight was collected in-person at baseline, 1 month, and 3 months using calibrated scales and via self-report at 6 months. We retrieved objective self-monitoring engagement data from MyFitnessPal using an application programming interface. Engagement was defined as the number of days per week in which tracking occurred, with diet entries counted if ≥800 kcal per day. Other assessment data were collected in-person via online self-report questionnaires.
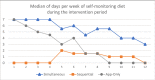
Results: At baseline, participants (84/100 female) had a mean age (SD) of 42.7 (11.7) years and a BMI of 31.9 (SD 4.5) kg/m2. One-third (33/100) were from racial or ethnic minority groups. During the trial, 5 participants became ineligible. Of the remaining 100 participants, 84% (84/100) and 76% (76/100) completed the 1-month and 3-month visits, respectively. In intent-to-treat analyses, there was no difference in weight change at 3 months between the Sequential arm (mean -2.7 kg, 95% CI -3.9 to -1.5) and either the App-Only arm (-2.4 kg, -3.7 to -1.2; P=.78) or the Simultaneous arm (-2.8 kg, -4.0 to -1.5; P=.72). The median number of days of self-monitoring diet per week was 1.9 (interquartile range [IQR] 0.3-5.5) in Sequential (once began), 5.3 (IQR 1.8-6.7) in Simultaneous, and 2.9 (IQR 1.2-5.2) in App-Only. Weight was tracked 4.8 (IQR 1.9-6.3) days per week in Sequential and 5.1 (IQR 1.8-6.3) days per week in Simultaneous. Engagement in neither diet nor weight tracking differed between arms.
Conclusions: Regardless of the order in which diet is tracked, using tailored goals and a commercial mobile app can produce clinically significant weight loss. Stand-alone digital health treatments may be a viable option for those looking for a lower intensity approach.
Trial registration: ClinicalTrials.gov NCT03254953; https://clinicaltrials.gov/ct2/show/NCT03254953 (Archived by WebCite at http://www.webcitation.org/72PyQrFjn).
Keywords: caloric restriction; mobile app; mobile health; obesity, self-monitoring; randomized controlled trial; technology; treatment adherence and compliance; weight loss.
©Michele L Patel, Christina M Hopkins, Taylor L Brooks, Gary G Bennett. Originally published in JMIR Mhealth and Uhealth (http://mhealth.jmir.org), 28.02.2019.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures


References
-
- Hartmann-Boyce J, Johns DJ, Jebb SA, Aveyard P, Behavioural Weight Management Review Group Effect of behavioural techniques and delivery mode on effectiveness of weight management: systematic review, meta-analysis and meta-regression. Obes Rev. 2014 Jul;15(7):598–609. doi: 10.1111/obr.12165. doi: 10.1111/obr.12165. - DOI - DOI - PMC - PubMed
-
- Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011 Jan;111(1):92–102. doi: 10.1016/j.jada.2010.10.008. http://europepmc.org/abstract/MED/21185970 S0002-8223(10)01644-5 - DOI - PMC - PubMed
-
- Lentferink AJ, Oldenhuis HK, de Groot M, Polstra L, Velthuijsen H, van Gemert-Pijnen JE. Key components in eHealth interventions combining self-tracking and persuasive eCoaching to promote a healthier lifestyle: a scoping review. J Med Internet Res. 2017 Aug 1;19(8):e277. doi: 10.2196/jmir.7288. http://www.jmir.org/2017/8/e277/ v19i8e277 - DOI - PMC - PubMed
-
- Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health. 2011 Nov;26(11):1479–98. doi: 10.1080/08870446.2010.540664.938640058 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

