Light-Induced Pulsed EPR Dipolar Spectroscopy on a Paradigmatic Hemeprotein
- PMID: 30817078
- PMCID: PMC6618045
- DOI: 10.1002/cphc.201900139
Light-Induced Pulsed EPR Dipolar Spectroscopy on a Paradigmatic Hemeprotein
Abstract
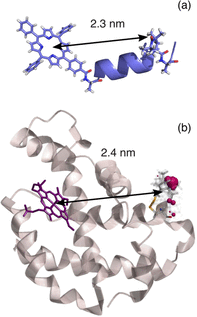
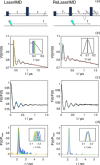
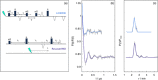
Light-induced pulsed EPR dipolar spectroscopic methods allow the determination of nanometer distances between paramagnetic sites. Here we employ orthogonal spin labels, a chromophore triplet state and a stable radical, to carry out distance measurements in singly nitroxide-labeled human neuroglobin. We demonstrate that Zn-substitution of neuroglobin, to populate the Zn(II) protoporphyrin IX triplet state, makes it possible to perform light-induced pulsed dipolar experiments on hemeproteins, extending the use of light-induced dipolar spectroscopy to this large class of metalloproteins. The versatility of the method is ensured by the employment of different techniques: relaxation-induced dipolar modulation enhancement (RIDME) is applied for the first time to the photoexcited triplet state. In addition, an alternative pulse scheme for laser-induced magnetic dipole (LaserIMD) spectroscopy, based on the refocused-echo detection sequence, is proposed for accurate zero-time determination and reliable distance analysis.
Keywords: DEER/PELDOR; EPR spectroscopy; heme proteins; porphyrinoids; triplet state.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Schiemann O., Prisner T. F., Q. Rev. Biophys. 2007, 40, 1-53. - PubMed
-
- Borbat P. P., Freed J. H., in Structural Information. from Spin-Labels and Intrinsic Paramagnetic Centres in Biosciences, (Eds.: C. R. Timmel, J. R. Harmer), Springer, Berlin Heidelberg, 2013, pp. 1–82.
-
- Banham J. E., Baker C. M., Ceola S., Day I. J., Grant G. H., Groenen E. J. J., Rodgers C. T., Jeschke G., Timmel C. R., J. Magn. Reson. 2008, 191, 202–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

