Salivary gland immunization via Wharton's duct activates differential T-cell responses within the salivary gland immune system
- PMID: 30817215
- PMCID: PMC6463922
- DOI: 10.1096/fj.201801993R
Salivary gland immunization via Wharton's duct activates differential T-cell responses within the salivary gland immune system
Abstract
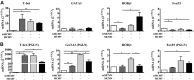
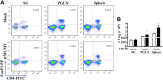
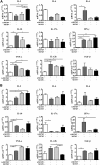
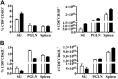
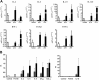
Salivary glands are a major component of the mucosal immune system that confer adaptive immunity to mucosal pathogens. As previously demonstrated, immunization of the submandibular gland with tissue culture-derived murine cytomegalovirus (tcMCMV) or replication-deficient adenoviruses expressing individual murine cytomegalovirus (MCMV) genes protected mice against a lethal MCMV challenge. Here, we report that salivary gland inoculation of BALB/cByJ mice with tcMCMV or recombinant adenoviruses differentially activates T helper (Th)1, -2, and -17 cells in the salivary glands vs. the associated lymph nodes. After inoculation with tcMCMV, lymphocytes from the submandibular gland preferentially express the transcription factor T-cell-specific T-box transcription factor (T-bet), which controls the expression of the hallmark Th1 cytokine, IFN-γ. Lymphocytes from the periglandular lymph nodes (PGLNs) express both T-bet and GATA-binding protein 3 (GATA3), which promotes the secretion of IL-4, -5, and -10 from Th2 cells. In contrast, after inoculation with replication-deficient adenoviruses, lymphocytes from the submandibular gland express T-bet, GATA3, and RAR-related orphan receptor γ, thymus-specific isoform (RORγt) (required for differentiation of Th17 cells) and forkhead box P3 (Foxp3) (required for the differentiation of regulatory T cells). Lymphocytes from the PGLNs were not activated. The differential induction of Th responses in the salivary gland vs. the PGLNs after inoculation with attenuated virus vs. a nominal protein antigen supports the use of the salivary as an alternative mucosal route for administering vaccines.-Liu, G., Zhang, F., Wang, R., London, S. D., London, L. Salivary gland immunization via Wharton's duct activates differential T-cell responses within the salivary gland immune system.
Keywords: T helper cells; mucosal immunity; vaccine development.
Conflict of interest statement
The authors thank Dr. John D. Shanley and Dr. Carol A. Wu (University of Connecticut, Farmington, CT, USA) for providing recombinant adenovirus vectors and cell lines. This project was supported by U.S. National Institutes of Health/National Institute of Dental and Craniofacial Research Grant 1R01 DE016652 (to S.D.L.) and 1R15DE024313 (to S.D.L.). The authors declare no conflicts of interest.
Figures

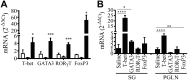
References
-
- Takahashi I., Nochi T., Yuki Y., Kiyono H. (2009) New horizon of mucosal immunity and vaccines. Curr. Opin. Immunol. 21, 352–358 - PubMed
-
- Brandtzaeg P. (2007) Induction of secretory immunity and memory at mucosal surfaces. Vaccine 25, 5467–5484 - PubMed
-
- Neutra M. R., Kozlowski P. A. (2006) Mucosal vaccines: the promise and the challenge. Nat. Rev. Immunol. 6, 148–158 - PubMed
-
- Tucker S. N., Lin K., Stevens S., Scollay R., Bennett M. J., Olson D. C. (2004) Salivary gland genetic vaccination: a scalable technology for promoting distal mucosal immunity and heightened systemic immune responses. Vaccine 22, 2500–2504 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

