Naturally presented HLA class I-restricted epitopes from the neurotrophic factor S100-β are targets of the autoimmune response in type 1 diabetes
- PMID: 30817223
- PMCID: PMC6463915
- DOI: 10.1096/fj.201802270R
Naturally presented HLA class I-restricted epitopes from the neurotrophic factor S100-β are targets of the autoimmune response in type 1 diabetes
Abstract
Type 1 diabetes (T1D) results from the destruction of pancreatic β-cells by the immune system, and CD8+ T lymphocytes are critical actors in this autoimmune response. Pancreatic islets are surrounded by a mesh of nervous cells, the peri-insular Schwann cells, which are also targeted by autoreactive T lymphocytes and express specific antigens, such as the neurotrophic factor S100-β. Previous work has shown increased proliferative responses to whole S100-β in both human T1D patients and the nonobese diabetic (NOD) mouse model. We describe for the first time naturally processed and presented epitopes (NPPEs) presented by class I human leukocyte antigen-A*02:01 (A2.1) molecules derived from S100-β. These NPPEs triggered IFN-γ responses more frequently in both newly diagnosed and long-term T1D patients compared with healthy donors. Furthermore, the same NPPEs are recognized during the autoimmune response leading to diabetes in A2.1-transgenic NOD mice as early as 4 wk of age. Interestingly, when these NPPEs are used to prevent diabetes in this animal model, an acceleration of the disease is observed together with an exacerbation in insulitis and an increase in S100-β-specific cytotoxicity in vaccinated animals. Whether these can be used in diabetes prevention needs to be carefully evaluated in animal models before use in future clinical assays.-Calviño-Sampedro, C., Gomez-Tourino, I., Cordero, O. J., Reche, P. A., Gómez-Perosanz, M., Sánchez-Trincado, J. L., Rodríguez, M. Á., Sueiro, A. M., Viñuela, J. E., Calviño, R. V. Naturally presented HLA class I-restricted epitopes from the neurotrophic factor S100-β are targets of the autoimmune response in type 1 diabetes.
Keywords: S100β peptide epitopes; autoantigen; cytotoxic lymphocytes; immunotherapy; peri-insular Schwann cells.
Conflict of interest statement
The authors thank Dr. Sefina Arif (King’s College London, London, United Kingdom) for critically reviewing the manuscript. This work was funded by the Ministerio de Economía y Competitividad (Grant BIO2014-53091-C3-3-R to R.V.C.). During this work, I.G.-T. was supported by a Maria Barbeito predoctoral fellowship (Xunta de Galicia, La Coruña, Spain). During this work, C.C.-S. was supported by a Deputación da Coruña grant (2012–2013 and 2016–2017). The authors declare no conflicts of interest.
Figures

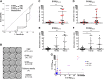
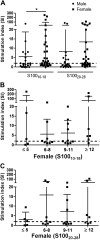


References
-
- Taplin C. E., Barker J. M. (2008) Autoantibodies in type 1 diabetes. Autoimmunity 41, 11–18 - PubMed
-
- Roep B. O., Peakman M. (2011) Diabetogenic T lymphocytes in human Type 1 diabetes. Curr. Opin. Immunol. 23, 746–753 - PubMed
-
- Anderson M. S., Bluestone J. A. (2005) The NOD mouse: a model of immune dysregulation. Annu. Rev. Immunol. 23, 447–485 - PubMed
-
- Liblau R. S., Wong F. S., Mars L. T., Santamaria P. (2002) Autoreactive CD8 T cells in organ-specific autoimmunity: emerging targets for therapeutic intervention. Immunity 17, 1–6 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

