Phase III Trial of PROSTVAC in Asymptomatic or Minimally Symptomatic Metastatic Castration-Resistant Prostate Cancer
- PMID: 30817251
- PMCID: PMC6494360
- DOI: 10.1200/JCO.18.02031
Phase III Trial of PROSTVAC in Asymptomatic or Minimally Symptomatic Metastatic Castration-Resistant Prostate Cancer
Abstract
Purpose: PROSTVAC, a viral vector-based immunotherapy, prolonged median overall survival (OS) by 8.5 months versus placebo in metastatic castration-resistant prostate cancer in a phase II study. This phase III study further investigated those findings.
Patients and methods: Patients were randomly assigned to PROSTVAC (Arm V; n = 432), PROSTVAC plus granulocyte-macrophage colony-stimulating factor (Arm VG; n = 432), or placebo (Arm P; n = 433), stratified by prostate-specific antigen (less than 50 ng/mL v 50 ng/mL or more) and lactate dehydrogenase (less than 200 v 200 U/L or more). Primary end point was OS. Secondary end points were patients alive without events (AWE)-namely, radiographic progression, pain progression, chemotherapy initiation, or death-at 6 months and safety. The study design was a superiority trial of PROSTVAC (Arm V or Arm VG) versus Arm P. Three interim analyses were planned.
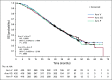
Results: At the third interim analysis, criteria for futility were met and the trial was stopped early. Neither active treatment had an effect on median OS (Arm V, 34.4 months; hazard ratio, 1.01; 95% CI, 0.84 to 1.20; P = .47; Arm VG, 33.2 months; hazard ratio, 1.02; 95% CI, 0.86 to 1.22; P = .59; Arm P, 34.3 months). Likewise, AWE at 6 months was similar (Arm V, 29.4%; odds ratio, 0.96; 95% CI, 0.71 to 1.29; Arm VG, 28.0%; odds ratio, 0.89; 95% CI, 0.66 to 1.20; placebo, 30.3%). Adverse events were similar for the treatment and placebo groups, with the most common being injection site reactions (62% to 72%) and fatigue (21% to 24%). Arrhythmias were the most common cardiac-related events (1.4% to 3.5%). There were no reports of either myocarditis or pericarditis. Serious treatment-related events occurred in less than 1% of all patients.
Conclusion: Whereas PROSTVAC was safe and well tolerated, it had no effect on OS or AWE in metastatic castration-resistant prostate cancer. Combination therapy is currently being explored in clinical trials.
Trial registration: ClinicalTrials.gov NCT01322490.
Figures
Comment in
-
Re: Phase III Trial of PROSTVAC in Asymptomatic or Minimally Symptomatic Metastatic Castration-resistant Prostate Cancer.Eur Urol. 2020 Jan;77(1):131-132. doi: 10.1016/j.eururo.2019.07.025. Epub 2019 Jul 24. Eur Urol. 2020. PMID: 31350066 No abstract available.
References
-
- Virgo KS, Basch E, Loblaw DA, et al. Second-line hormonal therapy for men with chemotherapy-naïve, castration-resistant prostate cancer: American Society of Clinical Oncology provisional clinical opinion. J Clin Oncol. 2017;35:1952–1964. - PubMed
-
- Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: Final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–992. Errata: Lancet Oncol 13:e464, 2012; Lancet Oncol 15:e365, 2014. - PubMed
-
- Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): Final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–160. - PubMed
-
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

