Extended diagnosis of purine and pyrimidine disorders from urine: LC MS/MS assay development and clinical validation
- PMID: 30817767
- PMCID: PMC6394934
- DOI: 10.1371/journal.pone.0212458
Extended diagnosis of purine and pyrimidine disorders from urine: LC MS/MS assay development and clinical validation
Abstract
Background and aims: Inborn errors of purine and pyrimidine metabolism are a diverse group of disorders with possible serious or life-threatening symptoms. They may be associated with neurological symptoms, renal stone disease or immunodeficiency. However, the clinical presentation can be nonspecific and mild so that a number of cases may be missed. Previously published assays lacked detection of certain diagnostically important biomarkers, including SAICAr, AICAr, beta-ureidoisobutyric acid, 2,8-dihydroxyadenine and orotidine, necessitating the use of separate assays for their detection. Moreover, the limited sensitivity for some analytes in earlier assays may have hampered the reliable detection of mild cases. Therefore, we aimed to develop a liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay that allows the simultaneous and sensitive detection of an extended range of purine and pyrimidine biomarkers in urine.
Methods: The assay was developed and validated using LC-MS/MS and clinically tested by analyzing ERNDIM Diagnostic Proficiency Testing (DPT) samples and further specimens from patients with various purine and pyrimidine disorders.
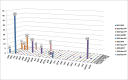
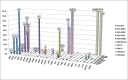
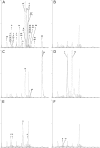
Results: Reliable determination of 27 analytes including SAICAr, AICAr, beta-ureidoisobutyric acid, 2,8-dihydroxyadenine and orotidine was achieved in urine following a simple sample preparation. The method clearly distinguished pathological and normal samples and differentiated between purine and pyrimidine defects in all clinical specimens.
Conclusions: A LC-MS/MS assay allowing the simultaneous, sensitive and reliable diagnosis of an extended range of purine and pyrimidine disorders has been developed. The validated method has successfully been tested using ERNDIM Diagnostic Proficiency Testing (DPT) samples and further clinical specimens from patients with various purine and pyrimidine disorders. Sample preparation is simple and assay duration is short, facilitating an easier inclusion of the assay into the diagnostic procedures.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Screening for adenylosuccinate lyase deficiency using tandem mass spectrometry analysis of succinylpurines in neonatal dried blood spots.Clin Biochem. 2015 Jan;48(1-2):2-7. doi: 10.1016/j.clinbiochem.2014.10.004. Epub 2014 Oct 23. Clin Biochem. 2015. PMID: 25445730
-
The need for vigilance: false-negative screening for adenylosuccinate lyase deficiency caused by deribosylation of urinary biomarkers.Clin Biochem. 2013 Dec;46(18):1899-901. doi: 10.1016/j.clinbiochem.2013.10.018. Epub 2013 Oct 30. Clin Biochem. 2013. PMID: 24183879
-
Comprehensive detection of disorders of purine and pyrimidine metabolism by HPLC with electrospray ionization tandem mass spectrometry.Clin Chem. 2006 Jun;52(6):1127-37. doi: 10.1373/clinchem.2005.058842. Epub 2006 Apr 13. Clin Chem. 2006. PMID: 16613999
-
[Phosphoribosyltransferase (APRT) deficiency--molecular and clinical aspects of dihydroxyadeninuria].Postepy Hig Med Dosw. 1998;52(1):89-104. Postepy Hig Med Dosw. 1998. PMID: 9608233 Review. Polish.
-
Uric acid changes in urine and plasma: an effective tool in screening for purine inborn errors of metabolism and other pathological conditions.J Inherit Metab Dis. 2007 Jun;30(3):295-309. doi: 10.1007/s10545-007-0455-8. Epub 2007 May 19. J Inherit Metab Dis. 2007. PMID: 17520339 Review.
Cited by
-
Structure and Elastic Properties of an Unsymmetrical Bi-Heterocyclic Azo Compound in the Langmuir Monolayer and Langmuir-Blodgett Film.ACS Omega. 2020 Aug 19;5(34):21538-21549. doi: 10.1021/acsomega.0c02147. eCollection 2020 Sep 1. ACS Omega. 2020. PMID: 32905463 Free PMC article.
-
The Urinary Metabolome of Newborns with Perinatal Complications.Metabolites. 2024 Jan 10;14(1):41. doi: 10.3390/metabo14010041. Metabolites. 2024. PMID: 38248844 Free PMC article.
-
Extending inherited metabolic disorder diagnostics with biomarker interaction visualizations.Orphanet J Rare Dis. 2023 Apr 26;18(1):95. doi: 10.1186/s13023-023-02683-9. Orphanet J Rare Dis. 2023. PMID: 37101200 Free PMC article.
-
Atypical presentation of Arts syndrome due to a novel hemizygous loss-of-function variant in the PRPS1 gene.Mol Genet Metab Rep. 2020 Nov 18;25:100677. doi: 10.1016/j.ymgmr.2020.100677. eCollection 2020 Dec. Mol Genet Metab Rep. 2020. PMID: 33294372 Free PMC article.
-
The Strange Case of Orotic Acid: The Different Expression of Pyrimidines Biosynthesis in Healthy Males and Females.J Pers Med. 2023 Sep 28;13(10):1443. doi: 10.3390/jpm13101443. J Pers Med. 2023. PMID: 37888054 Free PMC article.
References
-
- Bakker JA, Bierau J. Purine and pyrimidine metabolism: still more to learn. Ned Tijdschr Klin Chem Labgeneesk. 2012; 37: 3–6.
-
- Rebollido-Fernandez MM, Castiñeiras DE, Bóveda MD, Couce ML, Cocho JA, Fraga JM. Development of electrospray ionization tandem mass spectrometry methods for the study of a high number of urine markers of inborn errors of metabolism. Rapid Commun Mass Spectrom. 2012; 26: 2131–2144. 10.1002/rcm.6325 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

