Emergence of antibiotic resistance in immunocompromised host populations: A case study of emerging antibiotic resistant tuberculosis in AIDS patients
- PMID: 30817798
- PMCID: PMC6394933
- DOI: 10.1371/journal.pone.0212969
Emergence of antibiotic resistance in immunocompromised host populations: A case study of emerging antibiotic resistant tuberculosis in AIDS patients
Abstract
Objective: The evolution of antibiotic resistance is far outpacing the development of new antibiotics, causing global public health concern about infections that will increasingly be unresponsive to antimicrobials. This risk of emerging antibiotic resistance may be meaningfully altered in highly AIDS-immunocompromised populations. Such populations fundamentally alter the bacterial evolutionary landscape in two ways, which we seek to model and analyze. First, widespread, population-level immunoincompetence creates a novel host environment with disrupted selective pressures. Second, within AIDS-prevalent populations, the recommendation that antibiotics be taken to treat and prevent opportunistic infection raises the risk of selection for drug-resistant pathogens.
Design: To determine the impact of HIV/AIDS on the emergence of antibiotic resistance-specifically in the developing world where high prevalence and economic challenges complicate disease management.
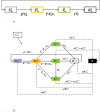
Methods: We present an SEIR epidemiological model of bacterial infection, and parametrize it to capture HIV/AIDS-attributable emergence of resistance under conditions of both high and low HIV/AIDS prevalence.
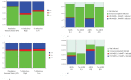
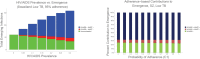
Results: We demonstrate that HIV/AIDS-immunocompromised hosts can be responsible for a disproportionately greater contribution to emergence of resistance than would be expected based on population-wide HIV/AIDS prevalence alone.
Conclusions: As such, the AIDS-immunocompromised have the potential become wellsprings of novel, resistant, opportunistic pathogen strains that can propagate into the broader global community. We discuss how public health policies for HIV/AIDS management can shape the evolutionary environment for opportunistic bacterial infections.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Masur PbH, Kaplan JE, Holmes KK. Guidelines for Preventing Opportunistic Infections among HIV-Infected Persons—2002: Recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America*. Annals of Internal Medicine. 2002;137(2):435–78. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

