Neoadjuvant therapy versus upfront surgery for potentially resectable pancreatic cancer: A Markov decision analysis
- PMID: 30817807
- PMCID: PMC6394923
- DOI: 10.1371/journal.pone.0212805
Neoadjuvant therapy versus upfront surgery for potentially resectable pancreatic cancer: A Markov decision analysis
Abstract
Background: Neoadjuvant therapy has emerged as an alternative treatment strategy for potentially resectable pancreatic cancer. In the absence of large randomized controlled trials offering a direct comparison, this study aims to use Markov decision analysis to compare efficacy of traditional surgery first (SF) and neoadjuvant treatment (NAT) pathways for potentially resectable pancreatic cancer.
Methods: An advanced Markov decision analysis model was constructed to compare SF and NAT pathways for potentially resectable pancreatic cancer. Transition probabilities were calculated from randomized control and Phase II/III trials after comprehensive literature search. Utility outcomes were measured in overall and quality-adjusted life months (QALMs) on an intention-to-treat basis as the primary outcome. Markov cohort analysis of treatment received was the secondary outcome. Model uncertainties were tested with one and two-way deterministic and probabilistic Monte Carlo sensitivity analysis.
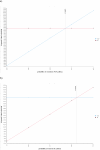
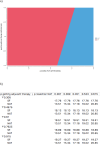
Results: SF gave 23.72 months (18.51 QALMs) versus 20.22 months (16.26 QALMs). Markov Cohort Analysis showed that where all treatment modalities were received NAT gave 35.05 months (29.87 QALMs) versus 30.96 months (24.86QALMs) for R0 resection and 34.08 months (29.87 QALMs) versus 25.85 months (20.72 QALMs) for R1 resection. One-way deterministic sensitivity analysis showed that NAT was superior if the resection rate was greater than 51.04% or below 75.68% in SF pathway. Two-way sensitivity analysis showed that pathway superiority depended on obtaining multimodal treatment in either pathway.
Conclusion: Whilst NAT is a viable alternative to traditional SF approach, superior pathway selection depends on the individual patient's likelihood of receiving multimodal treatment in either pathway.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Optimal management of patients with operable pancreatic head cancer: A Markov decision analysis.J Surg Oncol. 2021 Oct;124(5):801-809. doi: 10.1002/jso.26589. Epub 2021 Jul 7. J Surg Oncol. 2021. PMID: 34231222 Free PMC article.
-
The cost-effectiveness of neoadjuvant chemoradiation is superior to a surgery-first approach in the treatment of pancreatic head adenocarcinoma.Ann Surg Oncol. 2013 Dec;20 Suppl 3:S500-8. doi: 10.1245/s10434-013-2882-0. Epub 2013 Feb 10. Ann Surg Oncol. 2013. PMID: 23397153
-
Neoadjuvant therapy versus upfront surgical strategies in resectable pancreatic cancer: A Markov decision analysis.Eur J Surg Oncol. 2016 Oct;42(10):1552-60. doi: 10.1016/j.ejso.2016.07.016. Epub 2016 Aug 9. Eur J Surg Oncol. 2016. PMID: 27570116
-
Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer.Br J Surg. 2018 Jul;105(8):946-958. doi: 10.1002/bjs.10870. Epub 2018 Apr 30. Br J Surg. 2018. PMID: 29708592 Free PMC article. Review.
-
Systemic neoadjuvant chemotherapy in modern pancreatic cancer treatment: a systematic review and meta-analysis.Ann R Coll Surg Engl. 2019 Sep;101(7):453-462. doi: 10.1308/rcsann.2019.0060. Epub 2019 Jul 15. Ann R Coll Surg Engl. 2019. PMID: 31304767 Free PMC article.
Cited by
-
Essential updates 2018/2019: Current topics in the surgical treatment of pancreatic ductal adenocarcinoma.Ann Gastroenterol Surg. 2020 Aug 9;5(1):7-23. doi: 10.1002/ags3.12379. eCollection 2021 Jan. Ann Gastroenterol Surg. 2020. PMID: 33532676 Free PMC article. Review.
-
Development of a Biomarker-Based Scoring System Predicting Early Recurrence of Resectable Pancreatic Duct Adenocarcinoma.Ann Surg Oncol. 2022 Feb;29(2):1281-1293. doi: 10.1245/s10434-021-10866-6. Epub 2021 Oct 4. Ann Surg Oncol. 2022. PMID: 34608555 Free PMC article.
-
Neoadjuvant Chemotherapy-Chemoradiation for Borderline-Resectable Pancreatic Adenocarcinoma: A UK Tertiary Surgical Oncology Centre Series.Cancers (Basel). 2022 Sep 26;14(19):4678. doi: 10.3390/cancers14194678. Cancers (Basel). 2022. PMID: 36230600 Free PMC article.
-
Optimal management of patients with operable pancreatic head cancer: A Markov decision analysis.J Surg Oncol. 2021 Oct;124(5):801-809. doi: 10.1002/jso.26589. Epub 2021 Jul 7. J Surg Oncol. 2021. PMID: 34231222 Free PMC article.
References
-
- Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, et al. Adjuvant chemoradio- therapy and chemotherapy in resectable pancreatic cancer: a randomized controlled trial. Lancet. 2001;358:1576–85. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

