IMI - Report on Experimental Models of Emmetropization and Myopia
- PMID: 30817827
- PMCID: PMC6738517
- DOI: 10.1167/iovs.18-25967
IMI - Report on Experimental Models of Emmetropization and Myopia
Abstract
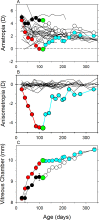
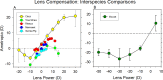
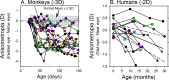
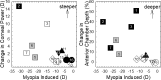
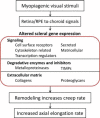
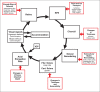
The results of many studies in a variety of species have significantly advanced our understanding of the role of visual experience and the mechanisms of postnatal eye growth, and the development of myopia. This paper surveys and reviews the major contributions that experimental studies using animal models have made to our thinking about emmetropization and development of myopia. These studies established important concepts informing our knowledge of the visual regulation of eye growth and refractive development and have transformed treatment strategies for myopia. Several major findings have come from studies of experimental animal models. These include the eye's ability to detect the sign of retinal defocus and undergo compensatory growth, the local retinal control of eye growth, regulatory changes in choroidal thickness, and the identification of components in the biochemistry of eye growth leading to the characterization of signal cascades regulating eye growth and refractive state. Several of these findings provided the proofs of concepts that form the scientific basis of new and effective clinical treatments for controlling myopia progression in humans. Experimental animal models continue to provide new insights into the cellular and molecular mechanisms of eye growth control, including the identification of potential new targets for drug development and future treatments needed to stem the increasing prevalence of myopia and the vision-threatening conditions associated with this disease.
Figures


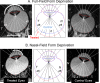

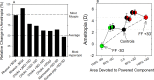
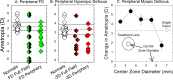

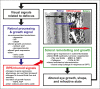
References
-
- Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036–1042. - PubMed
-
- Turnbull PR, Backhouse S, Phillips JR. Visually guided eye growth in the squid. Curr Biol. 2015;25:R791–R792. - PubMed
-
- Walls GL. The Vertebrate Eye and its Adaptive Radiations Hafner Publishing 1970 ed. Bloomfield Hills: Cranbrook Institute of Science;; 1942.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

