GenomeGraphR: A user-friendly open-source web application for foodborne pathogen whole genome sequencing data integration, analysis, and visualization
- PMID: 30818354
- PMCID: PMC6394949
- DOI: 10.1371/journal.pone.0213039
GenomeGraphR: A user-friendly open-source web application for foodborne pathogen whole genome sequencing data integration, analysis, and visualization
Abstract
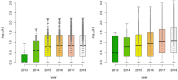
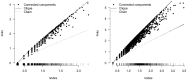
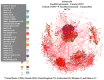
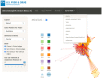
Food safety risk assessments and large-scale epidemiological investigations have the potential to provide better and new types of information when whole genome sequence (WGS) data are effectively integrated. Today, the NCBI Pathogen Detection database WGS collections have grown significantly through improvements in technology, coordination, and collaboration, such as the GenomeTrakr and PulseNet networks. However, high-quality genomic data is not often coupled with high-quality epidemiological or food chain metadata. We have created a set of tools for cleaning, curation, integration, analysis and visualization of microbial genome sequencing data. It has been tested using Salmonella enterica and Listeria monocytogenes data sets provided by NCBI Pathogen Detection (160,000 sequenced isolates in 2018). GenomeGraphR presents foodborne pathogen WGS data and associated curated metadata in a user-friendly interface that allows a user to query a variety of research questions such as, transmission sources and dynamics, global reach, and persistence of genotypes associated with contamination in the food supply and foodborne illness across time or space. The application is freely available (https://fda-riskmodels.foodrisk.org/genomegraphr/).
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







Similar articles
-
Implementation of Nationwide Real-time Whole-genome Sequencing to Enhance Listeriosis Outbreak Detection and Investigation.Clin Infect Dis. 2016 Aug 1;63(3):380-6. doi: 10.1093/cid/ciw242. Epub 2016 Apr 18. Clin Infect Dis. 2016. PMID: 27090985 Free PMC article.
-
Outbreak of Listeria monocytogenes in South Africa, 2017-2018: Laboratory Activities and Experiences Associated with Whole-Genome Sequencing Analysis of Isolates.Foodborne Pathog Dis. 2019 Jul;16(7):524-530. doi: 10.1089/fpd.2018.2586. Epub 2019 May 10. Foodborne Pathog Dis. 2019. PMID: 31062992 Free PMC article.
-
Tracking of Listeria monocytogenes in meat establishment using Whole Genome Sequencing as a food safety management tool: A proof of concept.Int J Food Microbiol. 2017 Sep 18;257:157-164. doi: 10.1016/j.ijfoodmicro.2017.06.015. Epub 2017 Jun 20. Int J Food Microbiol. 2017. PMID: 28666130
-
Whole genome sequencing uses for foodborne contamination and compliance: Discovery of an emerging contamination event in an ice cream facility using whole genome sequencing.Infect Genet Evol. 2019 Sep;73:214-220. doi: 10.1016/j.meegid.2019.04.026. Epub 2019 Apr 27. Infect Genet Evol. 2019. PMID: 31039448 Review.
-
Emerging needs and opportunities in foodborne disease detection and prevention: From tools to people.Food Microbiol. 2018 Oct;75:65-71. doi: 10.1016/j.fm.2017.07.006. Epub 2017 Jul 11. Food Microbiol. 2018. PMID: 30056965 Review.
Cited by
-
Prospective modeling and estimating the epidemiologically informative match rate within large foodborne pathogen genomic databases.BMC Res Notes. 2024 Jul 9;17(1):191. doi: 10.1186/s13104-024-06847-z. BMC Res Notes. 2024. PMID: 38982485 Free PMC article.
-
Use of whole genome sequencing for surveillance and control of foodborne diseases: status quo and quo vadis.Front Microbiol. 2024 Sep 13;15:1460335. doi: 10.3389/fmicb.2024.1460335. eCollection 2024. Front Microbiol. 2024. PMID: 39345263 Free PMC article. Review.
-
Listeriosis outbreak likely due to contaminated liver pâté consumed in a tavern, Austria, December 2018.Euro Surveill. 2019 Sep;24(39):1900274. doi: 10.2807/1560-7917.ES.2019.24.39.1900274. Euro Surveill. 2019. PMID: 31576804 Free PMC article.
-
Integrating Whole-Genome Sequencing Data Into Quantitative Risk Assessment of Foodborne Antimicrobial Resistance: A Review of Opportunities and Challenges.Front Microbiol. 2019 May 21;10:1107. doi: 10.3389/fmicb.2019.01107. eCollection 2019. Front Microbiol. 2019. PMID: 31231317 Free PMC article. Review.
-
Human Listeriosis.Clin Microbiol Rev. 2023 Mar 23;36(1):e0006019. doi: 10.1128/cmr.00060-19. Epub 2022 Dec 8. Clin Microbiol Rev. 2023. PMID: 36475874 Free PMC article. Review.
References
-
- Byrne L, Adams N, Glen K, Dallman TJ, Kar-Purkayastha I, Beasley G, et al. Epidemiological and Microbiological Investigation of an Outbreak of Severe Disease from Shiga Toxin-Producing Escherichia coli O157 Infection Associated with Consumption of a Slaw Garnish. J Food Prot. 2016;79(7):1161–8. Epub 2016/07/01. 10.4315/0362-028X.JFP-15-580 . - DOI - PubMed
-
- Franz E, Gras LM, Dallman T. Significance of whole genome sequencing for surveillance, source attribution and microbial risk assessment of foodborne pathogens. Current Opinion in Food Science. 2016;8:74–9. 10.1016/j.cofs.2016.04.004. - DOI
-
- Octavia S, Wang Q, Tanaka MM, Kaur S, Sintchenko V, Lan R. Delineating community outbreaks of Salmonella enterica serovar Typhimurium by use of whole-genome sequencing: insights into genomic variability within an outbreak. J Clin Microbiol. 2015;53(4):1063–71. Epub 2015/01/23. 10.1128/JCM.03235-14 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

