Sensing of cell-associated HTLV by plasmacytoid dendritic cells is regulated by dense β-galactoside glycosylation
- PMID: 30818370
- PMCID: PMC6413949
- DOI: 10.1371/journal.ppat.1007589
Sensing of cell-associated HTLV by plasmacytoid dendritic cells is regulated by dense β-galactoside glycosylation
Abstract
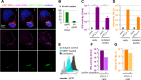
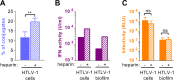
Human T Lymphotropic virus (HTLV) infection can persist in individuals resulting, at least in part, from viral escape of the innate immunity, including inhibition of type I interferon response in infected T-cells. Plasmacytoid dendritic cells (pDCs) are known to bypass viral escape by their robust type I interferon production. Here, we demonstrated that pDCs produce type I interferons upon physical cell contact with HTLV-infected cells, yet pDC activation inversely correlates with the ability of the HTLV-producing cells to transmit infection. We show that pDCs sense surface associated-HTLV present with glycan-rich structure referred to as biofilm-like structure, which thus represents a newly described viral structure triggering the antiviral response by pDCs. Consistently, heparan sulfate proteoglycans and especially the cell surface pattern of terminal β-galactoside glycosylation, modulate the transmission of the immunostimulatory RNA to pDCs. Altogether, our results uncover a function of virus-containing cell surface-associated glycosylated structures in the activation of innate immunity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Bruhn RL, Mahieux R, Murphy EL. Human lymphotropic viruses: HTLV-1 and HTLV-2 Clinical Virology 4th edition, ASM press; 2017.
-
- Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50: 481–92. - PubMed
-
- Gessain A, Barin F, Vernant J, Gout O, Maurs L, Calender A, et al. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2: 407–410. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

