A Review of Curcumin and Its Derivatives as Anticancer Agents
- PMID: 30818786
- PMCID: PMC6429287
- DOI: 10.3390/ijms20051033
A Review of Curcumin and Its Derivatives as Anticancer Agents
Abstract
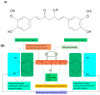
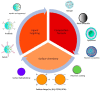
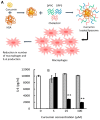
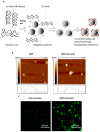
Cancer is the second leading cause of death in the world and one of the major public health problems. Despite the great advances in cancer therapy, the incidence and mortality rates of cancer remain high. Therefore, the quest for more efficient and less toxic cancer treatment strategies is still at the forefront of current research. Curcumin, the active ingredient of the Curcuma longa plant, has received great attention over the past two decades as an antioxidant, anti-inflammatory, and anticancer agent. In this review, a summary of the medicinal chemistry and pharmacology of curcumin and its derivatives in regard to anticancer activity, their main mechanisms of action, and cellular targets has been provided based on the literature data from the experimental and clinical evaluation of curcumin in cancer cell lines, animal models, and human subjects. In addition, the recent advances in the drug delivery systems for curcumin delivery to cancer cells have been highlighted.
Keywords: anticancer; cellular pathway; curcumin; delivery system; mechanism of action; structure activity relationship.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Gupta A.P., Pandotra P., Sharma R., Kushwaha M., Gupta S. Studies in Natural Products Chemistry. Volume 40. Elsevier; Amsterdam, The Netherlands: 2013. Chapter 8—Marine Resource: A Promising Future for Anticancer Drugs; pp. 229–325.
-
- Gupta A.P., Khan S., Manzoor M.M., Yadav A.K., Sharma G., Anand R., Gupta S. Chapter 10—Anticancer Curcumin: Natural Analogues and Structure-Activity Relationship. In: Atta ur R., editor. Studies in Natural Products Chemistry. Volume 54. Elsevier; Amsterdam, The Netherlands: 2017. pp. 355–401.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

