Nanomaterials-Based Colorimetric Immunoassays
- PMID: 30818816
- PMCID: PMC6473401
- DOI: 10.3390/nano9030316
Nanomaterials-Based Colorimetric Immunoassays
Abstract
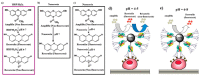
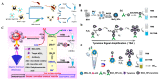
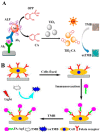
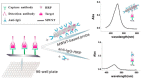
Colorimetric immunoassays for tumor marker detection have attracted considerable attention due to their simplicity and high efficiency. With the achievements of nanotechnology and nanoscience, nanomaterials-based colorimetric immunoassays have been demonstrated to be promising alternatives to conventional colorimetric enzyme-linked immunoassays. This review is focused on the progress in colorimetric immunoassays with the signal amplification of nanomaterials, including nanomaterials-based artificial enzymes to catalyze the chromogenic reactions, analyte-induced aggregation or size/morphology change of nanomaterials, nanomaterials as the carriers for loading enzyme labels, and chromogenic reactions induced by the constituent elements released from nanomaterials.
Keywords: colorimetric immunoassays; metal ions; nanomaterials; nanoparticle aggregation; nanozymes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
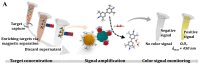
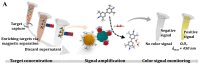












References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

