Targeting Ovarian Cancer Cells Overexpressing CD44 with Immunoliposomes Encapsulating Glycosylated Paclitaxel
- PMID: 30818864
- PMCID: PMC6429518
- DOI: 10.3390/ijms20051042
Targeting Ovarian Cancer Cells Overexpressing CD44 with Immunoliposomes Encapsulating Glycosylated Paclitaxel
Abstract
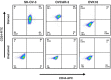
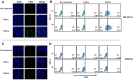
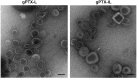
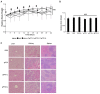
Paclitaxel (PTX) is one of the front-line drugs approved for the treatment of ovarian cancer. However, the application of PTX is limited due to the significant hydrophobicity and poor pharmacokinetics. We previously reported target-directed liposomes carrying tumor-selective conjugated antibody and encapsulated glycosylated PTX (gPTX-L) which successfully overcome the PTX limitation. The tubulin stabilizing activity of gPTX was equivalent to that of PTX while the cytotoxic activity of gPTX was reduced. In human ovarian cancer cell lines, SK-OV-3 and OVK18, the concentration at which cell growth was inhibited by 50% (IC50) for gPTX range from 15⁻20 nM, which was sensitive enough to address gPTX-L with tumor-selective antibody coupling for ovarian cancer therapy. The cell membrane receptor CD44 is associated with cancer progression and has been recognized as a cancer stem cell marker including ovarian cancer, becoming a suitable candidate to be targeted by gPTX-L therapy. In this study, gPTX-loading liposomes conjugated with anti-CD44 antibody (gPTX-IL) were assessed for the efficacy of targeting CD44-positive ovarian cancer cells. We successfully encapsulated gPTX into liposomes with the loading efficiency (LE) more than 80% in both of gPTX-L and gPTX-IL with a diameter of approximately 100 nm with efficacy of enhanced cytotoxicity in vitro and of convenient treatment in vivo. As the result, gPTX-IL efficiently suppressed tumor growth in vivo. Therefore gPTX-IL could be a promising formulation for effective ovarian cancer therapies.
Keywords: CD44; glycosylated paclitaxel; liposome; modified paclitaxel; ovarian cancer; specific targeting.
Conflict of interest statement
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of the manuscript entitled.
Figures




Similar articles
-
Paclitaxel-loaded hyaluronan solid nanoemulsions for enhanced treatment efficacy in ovarian cancer.Int J Nanomedicine. 2017 Jan 17;12:645-658. doi: 10.2147/IJN.S124158. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28176896 Free PMC article.
-
CD44-Targeting PLGA Nanoparticles Incorporating Paclitaxel and FAK siRNA Overcome Chemoresistance in Epithelial Ovarian Cancer.Cancer Res. 2018 Nov 1;78(21):6247-6256. doi: 10.1158/0008-5472.CAN-17-3871. Epub 2018 Aug 16. Cancer Res. 2018. PMID: 30115698
-
Hyaluronan oligomers-HPMA copolymer conjugates for targeting paclitaxel to CD44-overexpressing ovarian carcinoma.Pharm Res. 2012 Apr;29(4):1121-33. doi: 10.1007/s11095-012-0672-1. Epub 2012 Feb 16. Pharm Res. 2012. PMID: 22350800
-
Role of versican, hyaluronan and CD44 in ovarian cancer metastasis.Int J Mol Sci. 2011 Jan 31;12(2):1009-29. doi: 10.3390/ijms12021009. Int J Mol Sci. 2011. PMID: 21541039 Free PMC article. Review.
-
Innovative therapy of ovarian cancer based on overexpression of CD44 receptor.Ginekol Pol. 2015 May;86(5):388-91. doi: 10.17772/gp/2428. Ginekol Pol. 2015. PMID: 26117979 Review.
Cited by
-
Cholesterol Synthesis Is Important for Breast Cancer Cell Tumor Sphere Formation and Invasion.Biomedicines. 2022 Aug 6;10(8):1908. doi: 10.3390/biomedicines10081908. Biomedicines. 2022. PMID: 36009455 Free PMC article.
-
A landscape of recent advances in lipid nanoparticles and their translational potential for the treatment of solid tumors.Bioeng Transl Med. 2023 Nov 9;9(2):e10601. doi: 10.1002/btm2.10601. eCollection 2024 Mar. Bioeng Transl Med. 2023. PMID: 38435821 Free PMC article. Review.
-
Drug resistance‑related gene targets and molecular mechanisms in the A2780/Taxol‑resistant epithelial ovarian cancer cell line.Oncol Lett. 2024 Mar 26;27(5):232. doi: 10.3892/ol.2024.14365. eCollection 2024 May. Oncol Lett. 2024. PMID: 38586210 Free PMC article.
-
CD44: A New Prognostic Marker in Colorectal Cancer?Cancers (Basel). 2024 Apr 19;16(8):1569. doi: 10.3390/cancers16081569. Cancers (Basel). 2024. PMID: 38672650 Free PMC article. Review.
-
A Journey to Reach the Ovary Using Next-Generation Technologies.Int J Mol Sci. 2023 Nov 22;24(23):16593. doi: 10.3390/ijms242316593. Int J Mol Sci. 2023. PMID: 38068916 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

