Exploring Secondary Metabolite Profiles of Stachybotrys spp. by LC-MS/MS
- PMID: 30818881
- PMCID: PMC6468463
- DOI: 10.3390/toxins11030133
Exploring Secondary Metabolite Profiles of Stachybotrys spp. by LC-MS/MS
Abstract
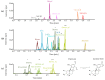
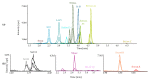
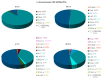
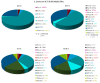
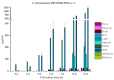
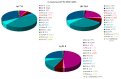
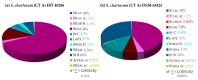
The genus Stachybotrys produces a broad diversity of secondary metabolites, including macrocyclic trichothecenes, atranones, and phenylspirodrimanes. Although the class of the phenylspirodrimanes is the major one and consists of a multitude of metabolites bearing various structural modifications, few investigations have been carried out. Thus, the presented study deals with the quantitative determination of several secondary metabolites produced by distinct Stachybotrys species for comparison of their metabolite profiles. For that purpose, 15 of the primarily produced secondary metabolites were isolated from fungal cultures and structurally characterized in order to be used as analytical standards for the development of an LC-MS/MS multimethod. The developed method was applied to the analysis of micro-scale extracts from 5 different Stachybotrys strains, which were cultured on different media. In that process, spontaneous dialdehyde/lactone isomerization was observed for some of the isolated secondary metabolites, and novel stachybotrychromenes were quantitatively investigated for the first time. The metabolite profiles of Stachybotrys species are considerably influenced by time of growth and substrate availability, as well as the individual biosynthetic potential of the respective species. Regarding the reported adverse effects associated with Stachybotrys growth in building environments, combinatory effects of the investigated secondary metabolites should be addressed and the role of the phenylspirodrimanes re-evaluated in future research.
Keywords: LC-MS/MS; Stachybotrys spp.; biosynthetic production; metabolite profiles; phenylspirodrimanes; satratoxins; stachybotrychromenes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
In Vitro Metabolism of Phenylspirodrimanes Derived from the Indoor Fungus Stachybotrys.Toxins (Basel). 2022 Jun 8;14(6):395. doi: 10.3390/toxins14060395. Toxins (Basel). 2022. PMID: 35737056 Free PMC article.
-
Artificial vs natural Stachybotrys infestation-Comparison of mycotoxin production on various building materials.Indoor Air. 2020 Nov;30(6):1268-1282. doi: 10.1111/ina.12705. Epub 2020 Jun 24. Indoor Air. 2020. PMID: 32510685
-
Mass spectrometry-based strategy for direct detection and quantification of some mycotoxins produced by Stachybotrys and Aspergillus spp. in indoor environments.Appl Environ Microbiol. 2007 Jul;73(13):4211-7. doi: 10.1128/AEM.00343-07. Epub 2007 May 4. Appl Environ Microbiol. 2007. PMID: 17483261 Free PMC article.
-
Stachybotrys chartarum: a fungus for our time.Phytochemistry. 2003 Sep;64(1):53-60. doi: 10.1016/s0031-9422(03)00275-9. Phytochemistry. 2003. PMID: 12946405 Review.
-
Stachybotrys chartarum-A Hidden Treasure: Secondary Metabolites, Bioactivities, and Biotechnological Relevance.J Fungi (Basel). 2022 May 12;8(5):504. doi: 10.3390/jof8050504. J Fungi (Basel). 2022. PMID: 35628759 Free PMC article. Review.
Cited by
-
Rapid and selective detection of macrocyclic trichothecene producing Stachybotrys chartarum strains by loop-mediated isothermal amplification (LAMP).Anal Bioanal Chem. 2021 Aug;413(19):4801-4813. doi: 10.1007/s00216-021-03436-y. Epub 2021 Jun 15. Anal Bioanal Chem. 2021. PMID: 34129076 Free PMC article.
-
Semisynthetic Approach toward Biologically Active Derivatives of Phenylspirodrimanes from S. chartarum.ACS Omega. 2022 Nov 28;7(49):45215-45230. doi: 10.1021/acsomega.2c05681. eCollection 2022 Dec 13. ACS Omega. 2022. PMID: 36530258 Free PMC article.
-
In Vitro Metabolism of Phenylspirodrimanes Derived from the Indoor Fungus Stachybotrys.Toxins (Basel). 2022 Jun 8;14(6):395. doi: 10.3390/toxins14060395. Toxins (Basel). 2022. PMID: 35737056 Free PMC article.
-
Biological evaluation of semi-synthetic isoindolinone isomers produced by Stachybotrys chartarum.Front Fungal Biol. 2024 Nov 22;5:1494795. doi: 10.3389/ffunb.2024.1494795. eCollection 2024. Front Fungal Biol. 2024. PMID: 39650337 Free PMC article.
-
Update on Stachybotrys chartarum-Black Mold Perceived as Toxigenic and Potentially Pathogenic to Humans.Biology (Basel). 2022 Feb 23;11(3):352. doi: 10.3390/biology11030352. Biology (Basel). 2022. PMID: 35336726 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

