Liposomal Curcumin is Better than Curcumin to Alleviate Complications in Experimental Diabetic Mellitus
- PMID: 30818888
- PMCID: PMC6429477
- DOI: 10.3390/molecules24050846
Liposomal Curcumin is Better than Curcumin to Alleviate Complications in Experimental Diabetic Mellitus
Abstract
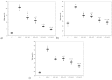
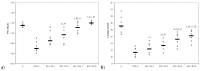
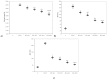
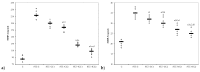
Curcumin (CC) is known to have anti-inflammatory and anti-oxidative properties and has already been tested for its efficiency in different diseases including diabetes mellitus (DM). New formulations and route administration were designed to obtain products with higher bioavailability. Our study aimed to test the effect of intraperitoneal (i.p.) administration of liposomal curcumin (lCC) as pre-treatment in streptozotocin(STZ)-induced DM in rats on oxidative stress, liver, and pancreatic functional parameters. Forty-two Wistar-Bratislava rats were randomly divided into six groups (seven animals/group): control (no diabetes), control-STZ (STZ-induced DM -60 mg/100g body weight a single dose intraperitoneal administration, and no CC pre-treatment), two groups with DM and CC pre-treatment (1mg/100g bw-STZ + CC1, 2 mg/100g bw-STZ + CC2), and two groups with DM and lCC pre-treatment (1 mg/100g bw-STZ + lCC1, 2 mg/100g bw-STZ + lCC1). Intraperitoneal administration of Curcumin in diabetic rats showed a significant reduction of nitric oxide, malondialdehyde, total oxidative stress, and catalase for both evaluated formulations (CC and lCC) compared to control group (p < 0.005), with higher efficacy of lCC formulation compared to CC solution (p < 0.002, excepting catalase for STZ + CC2vs. STZ + lCC1when p = 0.0845). The CC and lCC showed hepatoprotective and hypoglycemic effects, a decrease in oxidative stress and improvement in anti-oxidative capacity status against STZ-induced DM in rats (p < 0.002). The lCC also proved better efficacy on MMP-2, and -9 plasma levels as compared to CC (p < 0.003, excepting STZ + CC2 vs. STZ + lCC1 comparison with p = 0.0553). The lCC demonstrated significantly better efficacy as compared to curcumin solution on all serum levels of the investigated markers, sustaining its possible use as adjuvant therapy in DM.
Keywords: catalase; curcumin; diabetes mellitus (DM); malondialdehyde (MDA); matrix metalloproteinases (MMP); nitric oxide (NOx); oxidative stress; streptozotocin (STZ).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

