Fibroblast Primary Cilia Are Required for Cardiac Fibrosis
- PMID: 30818997
- PMCID: PMC6517085
- DOI: 10.1161/CIRCULATIONAHA.117.028752
Fibroblast Primary Cilia Are Required for Cardiac Fibrosis
Abstract
Background: The primary cilium is a singular cellular structure that extends from the surface of many cell types and plays crucial roles in vertebrate development, including that of the heart. Whereas ciliated cells have been described in developing heart, a role for primary cilia in adult heart has not been reported. This, coupled with the fact that mutations in genes coding for multiple ciliary proteins underlie polycystic kidney disease, a disorder with numerous cardiovascular manifestations, prompted us to identify cells in adult heart harboring a primary cilium and to determine whether primary cilia play a role in disease-related remodeling.
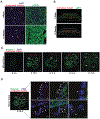
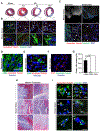
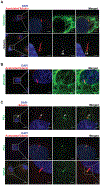
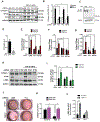
Methods: Histological analysis of cardiac tissues from C57BL/6 mouse embryos, neonatal mice, and adult mice was performed to evaluate for primary cilia. Three injury models (apical resection, ischemia/reperfusion, and myocardial infarction) were used to identify the location and cell type of ciliated cells with the use of antibodies specific for cilia (acetylated tubulin, γ-tubulin, polycystin [PC] 1, PC2, and KIF3A), fibroblasts (vimentin, α-smooth muscle actin, and fibroblast-specific protein-1), and cardiomyocytes (α-actinin and troponin I). A similar approach was used to assess for primary cilia in infarcted human myocardial tissue. We studied mice silenced exclusively in myofibroblasts for PC1 and evaluated the role of PC1 in fibrogenesis in adult rat fibroblasts and myofibroblasts.
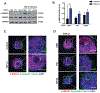
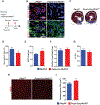
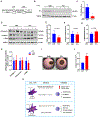
Results: We identified primary cilia in mouse, rat, and human heart, specifically and exclusively in cardiac fibroblasts. Ciliated fibroblasts are enriched in areas of myocardial injury. Transforming growth factor β-1 signaling and SMAD3 activation were impaired in fibroblasts depleted of the primary cilium. Extracellular matrix protein levels and contractile function were also impaired. In vivo, depletion of PC1 in activated fibroblasts after myocardial infarction impaired the remodeling response.
Conclusions: Fibroblasts in the neonatal and adult heart harbor a primary cilium. This organelle and its requisite signaling protein, PC1, are required for critical elements of fibrogenesis, including transforming growth factor β-1-SMAD3 activation, production of extracellular matrix proteins, and cell contractility. Together, these findings point to a pivotal role of this organelle, and PC1, in disease-related pathological cardiac remodeling and suggest that some of the cardiovascular manifestations of autosomal dominant polycystic kidney disease derive directly from myocardium-autonomous abnormalities.
Keywords: PKD1 protein; TGF-beta; cilia; fibroblasts; fibrosis; myocardial infarction.
Figures

References
-
- Gazea M, Tasouri E, Tolve M, Bosch V, Kabanova A, Gojak C, Kurtulmus B, Novikov O, Spatz J, Pereira G, Hubner W, Brodski C, Tucker KL and Blaess S. Primary cilia are critical for Sonic hedgehog-mediated dopaminergic neurogenesis in the embryonic midbrain. Dev Biol. 2016;409:55–71. DOI: 10.1016/j.ydbio.2015.10.033 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

