Transcriptomic profiling identifies differentially expressed genes associated with programmed cell death of nucellar cells in Ginkgo biloba L
- PMID: 30819114
- PMCID: PMC6396491
- DOI: 10.1186/s12870-019-1671-8
Transcriptomic profiling identifies differentially expressed genes associated with programmed cell death of nucellar cells in Ginkgo biloba L
Abstract
Background: Previously, we demonstrated that pollen chamber formation (PCF) in G. biloba ovules was a process of programmed cell death (PCD) within the nucellar cells at the micropylar end. However, the signal triggering the cascades of the programmed events in these nucellar cells remains unexplored.
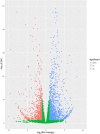
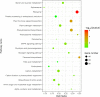
Results: A transcriptomic strategy was employed to unravel the mechanism underlying the nucellar PCD via the comparative profiles of RNA-seq between pre-PCF and post-PCF ovules. A total of 5599 differentially expressed genes (DEGs) with significance was identified from G. biloba ovules and classified into three main categories of GO annotation, including 17 biological processes, 15 cellular components and 17 molecular functions. KEGG analysis showed that 72 DEGs were enriched in "Plant hormone signal transduction". Furthermore, 99 DEGs were found to be associated with the PCD process, including the genes involved in ethylene signaling pathway, PCD initiation, and PCD execution. Moreover, calcium-cytochemical localization indicated that calcium could play a role in regulating PCD events within the nucellar cells during pollen chamber formation in G. biloba ovules.
Conclusions: A putative working model, consisting of three overlapping processes, is proposed for the nucellar PCD: at the stage of PCD preparation, ethylene signaling pathway is activated for transcriptional regulation of the downstream targets; subsequently, at the stage of PCD initiation, the upregulated expression of several transcription factors, i.e., NAC, bHLH, MADS-box, and MYB, further promotes the corresponding transcript levels of CYTOCHROME C and CALMODULINs, thereby, leads to the PCD initiation via the calcium-dependent signaling cascade; finally, at the stage of PCD execution, some proteases like metacaspases and vacuolar processing enzyme for hydrolysis, together with the process of autophagy, play roles in the clearance of cellular components. Afterwards, a pollen chamber is generated from the removal of specific nucellar cells in the developing ovule.
Keywords: Ginkgo biloba L.; Nucellus; Ovule; Pollen chamber; Programmed cell death (PCD); Transcriptomics.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures









Similar articles
-
Formation of archegonium chamber is associated with nucellar-cell programmed cell death in Ginkgo biloba.Protoplasma. 2007;231(3-4):173-81. doi: 10.1007/s00709-007-0257-8. Epub 2007 Aug 30. Protoplasma. 2007. PMID: 17762908
-
Identification and Characterization of MicroRNAs in Ginkgo biloba var. epiphylla Mak.PLoS One. 2015 May 15;10(5):e0127184. doi: 10.1371/journal.pone.0127184. eCollection 2015. PLoS One. 2015. PMID: 25978425 Free PMC article.
-
Genome-wide identification of the EIN3/EIL gene family in Ginkgo biloba and functional study of a GbEIL in the ethylene signaling pathway.Gene. 2024 Nov 30;928:148800. doi: 10.1016/j.gene.2024.148800. Epub 2024 Jul 25. Gene. 2024. PMID: 39067545
-
Plant life needs cell death, but does plant cell death need Cys proteases?FEBS J. 2017 May;284(10):1577-1585. doi: 10.1111/febs.14034. Epub 2017 Feb 26. FEBS J. 2017. PMID: 28165668 Review.
-
Hemoglobins, programmed cell death and somatic embryogenesis.Plant Sci. 2013 Oct;211:35-41. doi: 10.1016/j.plantsci.2013.06.010. Epub 2013 Jun 24. Plant Sci. 2013. PMID: 23987809 Review.
Cited by
-
Role of ethylene in the regulatory mechanism underlying the abortion of ovules after fertilization in Xanthoceras sorbifolium.Plant Mol Biol. 2021 May;106(1-2):67-84. doi: 10.1007/s11103-021-01130-2. Epub 2021 Feb 21. Plant Mol Biol. 2021. PMID: 33611782
-
Transcriptomic analysis of early fruit development in Chinese white pear (Pyrus bretschneideri Rehd.) and functional identification of PbCCR1 in lignin biosynthesis.BMC Plant Biol. 2019 Oct 11;19(1):417. doi: 10.1186/s12870-019-2046-x. BMC Plant Biol. 2019. PMID: 31604417 Free PMC article.
-
Characterization of the Calmodulin/Calmodulin-like Protein (CAM/CML) Family in Ginkgo biloba, and the Influence of an Ectopically Expressed GbCML Gene (Gb_30819) on Seedling and Fruit Development of Transgenic Arabidopsis.Plants (Basel). 2022 Jun 4;11(11):1506. doi: 10.3390/plants11111506. Plants (Basel). 2022. PMID: 35684283 Free PMC article.
-
Basis of single-seed formation in chestnut: cytomorphological observations reveal ovule developmental patterns of Castanea henryi.PeerJ. 2025 Jan 2;13:e18711. doi: 10.7717/peerj.18711. eCollection 2025. PeerJ. 2025. PMID: 39763705 Free PMC article.
-
Transcriptional Profiling of Resistant and Susceptible Cultivars of Grapevine (Vitis L.) Reveals Hypersensitive Responses to Plasmopara viticola.Front Microbiol. 2022 Apr 25;13:846504. doi: 10.3389/fmicb.2022.846504. eCollection 2022. Front Microbiol. 2022. PMID: 35572700 Free PMC article.
References
-
- Lee CL. Fertilization in Ginkgo biloba. Bot Gaz. 1955;117:79–100. doi: 10.1086/335894. - DOI
-
- Li DH, Yang X, Cui X, Cui KM, Li ZL. Early development of pollen chamber in Ginkgo biloba ovule. Acta Bot Sin. 2002;44:757–763.
-
- Li DH, Yang X, Cui KM, Li ZL. Morphological changes in nucellar cells undergoing programmed cell death (PCD) during pollen chamber formation in Ginkgo biloba. Acta Bot Sin. 2003;45:53–63.
MeSH terms
Substances
Grants and funding
- KJ2016A225/the Key University Science Research Project of Anhui Province, China
- 1808085MC58/the Natural Science Foundation of Anhui Province, China
- 2015-1098/the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, China
- 2015GXK015/the Provincial Quality Engineer Fund of Anhui Education Department, China
LinkOut - more resources
Full Text Sources

