The economic burden of bronchiectasis - known and unknown: a systematic review
- PMID: 30819166
- PMCID: PMC6393984
- DOI: 10.1186/s12890-019-0818-6
The economic burden of bronchiectasis - known and unknown: a systematic review
Abstract
Background: The increasing prevalence and recognition of bronchiectasis in clinical practice necessitates a better understanding of the economic disease burden to improve the management and achieve better clinical and economic outcomes. This study aimed to assess the economic burden of bronchiectasis based on a review of published literature.
Methods: A systematic literature review was conducted using MEDLINE, Embase, EconLit and Cochrane databases to identify publications (1 January 2001 to 31 December 2016) on the economic burden of bronchiectasis in adults.
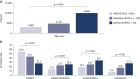
Results: A total of 26 publications were identified that reported resource use and costs associated with management of bronchiectasis. Two US studies reported annual incremental costs of bronchiectasis versus matched controls of US$5681 and US$2319 per patient. Twenty-four studies reported on hospitalization rates or duration of hospitalization for patients with bronchiectasis. Mean annual hospitalization rates per patient, reported in six studies, ranged from 0.3-1.3, while mean annual age-adjusted hospitalization rates, reported in four studies, ranged from 1.8-25.7 per 100,000 population. The average duration of hospitalization, reported in 12 studies, ranged from 2 to 17 days. Eight publications reported management costs of bronchiectasis. Total annual management costs of €3515 and €4672 per patient were reported in two Spanish studies. Two US studies reported total costs of approximately US$26,000 in patients without exacerbations, increasing to US$36,00-37,000 in patients with exacerbations. Similarly, a Spanish study reported higher total annual costs for patients with > 2 exacerbations per year (€7520) compared with those without exacerbations (€3892). P. aeruginosa infection increased management costs by US$31,551 to US$56,499, as reported in two US studies, with hospitalization being the main cost driver.
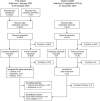
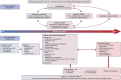
Conclusions: The current literature suggests that the economic burden of bronchiectasis in society is significant. Hospitalization costs are the major driver behind these costs, especially in patients with frequent exacerbations. However, the true economic burden of bronchiectasis is likely to be underestimated because most studies were retrospective, used ICD-9-CM coding to identify patients, and often ignored outpatient burden and cost. We present a conceptual framework to facilitate a more comprehensive assessment of the true burden of bronchiectasis for individuals, healthcare systems and society.
Keywords: Bronchiectasis; Burden of illness; Costs; Economic burden; Hospitalization; Resource use.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Dr. Goeminne reports receiving personal fees from Astra Zeneca, Pfizer, Novartis and Chiesi, and non-financial support from Boehringer and Bayer. None of the support received relates to the submitted work.
Juan Francisco Hernandez carried out the systematic review as an employee of Pharmerit, with funding from Bayer AG.
Roland Diel reports personal fees from Bayer, INSMED and Riemser Pharma. None of the support received relates to the submitted work.
Anna Filonenko is an employee of Bayer AG.
Rowena Hughes reports personal fees from highfield:communication for medical writing services.
Fabian Juelich is an employee of Bayer Vital GmbH.
George Solomon has nothing to disclose.
Alex Upton is an employee of Bayer plc.
Kamonthip Wichmann is an employee of Bayer AG.
Weiwei Xu carried out the systematic review as an employee of Pharmerit, with funding from Bayer AG.
James D Chalmers reports fees from Bayer Healthcare, during the conduct of the study; grants and personal fees from GlaxoSmithKline, Boehringer Ingelheim, Pfizer, Bayer Healthcare and Grifols, grants from AstraZeneca, and personal fees from Napp. None of the support received relates to the submitted work.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Vendrell M, de Gracia J, Olveira C, Martinez MA, Giron R, Maiz L, Canton R, Coll R, Escribano A, Sole A: Diagnosis and treatment of bronchiectasis. Spanish Society of Pneumology and Thoracic Surgery Arch Bronconeumol 2008, 44(11):629–640. - PubMed
-
- Chang AB, Bell SC, Torzillo PJ, King PT, Maguire GP, Byrnes CA, Holland AE, O'Mara P, Grimwood K, voting g e. Chronic suppurative lung disease and bronchiectasis in children and adults in Australia and new Zealand Thoracic Society of Australia and new Zealand guidelines. Med J Aust. 2015;202(1):21–23. doi: 10.5694/mja14.00287. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

