Resilience Is Associated With Larger Dentate Gyrus, While Suicide Decedents With Major Depressive Disorder Have Fewer Granule Neurons
- PMID: 30819514
- PMCID: PMC6830307
- DOI: 10.1016/j.biopsych.2018.12.022
Resilience Is Associated With Larger Dentate Gyrus, While Suicide Decedents With Major Depressive Disorder Have Fewer Granule Neurons
Abstract
Background: Early life adversity (ELA) increases major depressive disorder (MDD) and suicide risk and potentially affects dentate gyrus (DG) plasticity. We reported smaller DG and fewer granular neurons (GNs) in MDD. ELA effects on DG plasticity in suicide decedents with MDD (MDDSui) and resilient subjects (ELA history without MDD or suicide) are unknown.
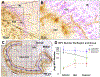
Methods: We quantified neural progenitor cells (NPCs), GNs, glia, and DG volume in whole hippocampus postmortem in four groups of drug-free, neuropathology-free subjects (N = 52 total): psychological autopsy-defined MDDSui and control subjects with and without ELA (before 15 years of age).
Results: ELA was associated with larger DG (p < .0001) and trending fewer NPCs (p = .0190) only in control subjects in whole DG, showing no effect on NPCs and DG volume in MDDSui. ELA exposure was associated with more GNs (p = .0003) and a trend for more glia (p = .0160) in whole DG in MDDSui and control subjects. MDDSui without ELA had fewer anterior and mid DG GNs (p < .0001), fewer anterior DG NPCs (p < .0001), and smaller whole DG volume (p = .0005) compared with control subjects without ELA. In MDDSui, lower Global Assessment Scale score correlated with fewer GNs and smaller DG.
Conclusions: Resilience to ELA involves a larger DG, perhaps related to more neurogenesis depleting NPCs, and because mature GNs and glia numbers do not differ in the resilient group, perhaps there are effects on process extension and synaptic load that can be examined in future studies. In MDDSui without ELA, smaller DG volume, with fewer GNs and NPCs, suggests less neurogenesis and/or more apoptosis and dendrite changes.
Keywords: Granule cell; Major depression; Neural progenitors; Postmortem; Stress; Suicide.
Copyright © 2019 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures


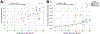
References
-
- Nelson EC, Heath AC, Madden PA, Cooper ML, Dinwiddie SH, Bucholz KK, et al. (2002): Association between self-reported childhood sexual abuse and adverse psychosocial outcomes: Results from a twin study. Arch Gen Psychiatry 59:139–145. - PubMed
-
- Roy A, Rylander G, Forslund K, Asberg M, Mazzanti CM, Goldman D, et al. (2001): Excess tryptophan hydroxylase 17 779C allele in surviving cotwins of monozygotic twin suicide victims. Neuropsychobiology 43:233–236. - PubMed
-
- Roy A, Sarchiapone M, Carli V (2007): Low resilience in suicide attempters: Relationship to depressive symptoms. Depress Anxiety 24:273–274. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

