Hematologic Complications of Immune Checkpoint Inhibitors
- PMID: 30819785
- PMCID: PMC6516131
- DOI: 10.1634/theoncologist.2018-0574
Hematologic Complications of Immune Checkpoint Inhibitors
Abstract
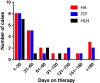
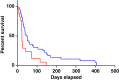
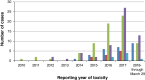
Immune checkpoint inhibitors have improved outcomes for patients with numerous hematological and solid cancers. Hematologic toxicities have been described, but the spectrum, timing, and clinical presentation of these complications are not well understood. We used the World Health Organization's pharmacovigilance database of individual-case-safety-reports (ICSRs) of adverse drug reactions, VigiBase, to identify cases of hematologic toxicities complicating immune checkpoint inhibitor therapy. We identified 168 ICSRs of immune thrombocytopenic purpura (ITP), hemolytic anemia (HA), hemophagocytic lymphohistiocytosis, aplastic anemia, and pure red cell aplasia in 164 ICSRs. ITP (n = 68) and HA (n = 57) were the most common of these toxicities and occurred concomitantly in four patients. These events occurred early on treatment (median 40 days) and were associated with fatal outcome in 12% of cases. Ipilimumab-based therapy (monotherapy or combination with anti-programmed death-1 [PD-1]) was associated with earlier onset (median 23 vs. 47.5 days, p = .006) than anti-PD-1/programmed death ligand-1 monotherapy. Reporting of hematologic toxicities has increased over the past 2 years (98 cases between January 2017 and March 2018 vs. 70 cases before 2017), possibly because of increased use of checkpoint inhibitors and improved recognition of toxicities. Future studies should evaluate incidence of hematologic toxicities, elucidate risk factors, and determine the most effective treatment algorithms. KEY POINTS: Immune-mediated hematologic toxicities are a potential side effect of immune checkpoint inhibitors (ICIs).Providers should monitor complete blood counts during treatment with ICIs.Corticosteroids are the mainstay of treatment for immune-mediated hematologic toxicities.Further research is needed to define patient-specific risk factors and optimal management strategies for hematologic toxicities.
© AlphaMed Press 2019.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Kong BY, Micklethwaite KP, Swaminathan S et al. Autoimmune hemolytic anemia induced by anti‐PD‐1 therapy in metastatic melanoma. Melanoma Res 2016;26:202–204. - PubMed
-
- Nair R, Gheith S, Nair SG. Immunotherapy‐associated hemolytic anemia with pure red‐cell aplasia. N Engl J Med 2016;374:1096–1097. - PubMed
-
- Tardy MP, Gastaud L, Boscagli A et al. Autoimmune hemolytic anemia after nivolumab treatment in Hodgkin lymphoma responsive to immunosuppressive treatment. A case report. Hematol Oncol 2017;35:875–877. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

