Burst Firing and Spatial Coding in Subicular Principal Cells
- PMID: 30819796
- PMCID: PMC6510334
- DOI: 10.1523/JNEUROSCI.1656-18.2019
Burst Firing and Spatial Coding in Subicular Principal Cells
Abstract
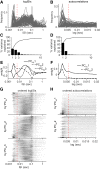
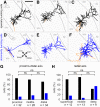
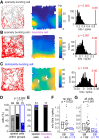
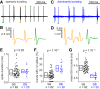
The subiculum is the major output structure of the hippocampal formation and is involved in learning and memory as well as in spatial navigation. Little is known about how neuronal diversity contributes to function in the subiculum. Previously, in vitro studies have identified distinct bursting patterns in the subiculum. Here, we asked how burst firing is related to spatial coding in vivo Using juxtacellular recordings in freely moving male rats, we studied the bursting behavior of 102 subicular principal neurons and distinguished two populations: sparsely bursting (∼80%) and dominantly bursting neurons (∼20%). These bursting behaviors were not linked to anatomy: both cell types were found all along the proximodistal and radial axes of the subiculum and all identified cells were pyramidal neurons. However, the distinct burst firing patterns were related to functional differences: the activity of sparsely bursting cells showed a stronger spatial modulation than the activity of dominantly bursting neurons. In addition, all cells classified as boundary cells were sparsely bursting cells. In most sparsely bursting cells, bursts defined sharper firing fields and carried more spatial information than isolated spikes. We conclude that burst firing is functionally relevant to subicular spatially tuned neurons, possibly by serving as a mechanism to transmit spatial information to downstream structures.SIGNIFICANCE STATEMENT The subiculum is the major output structure of the hippocampal formation and is involved in spatial navigation. In vitro, subicular cells can be distinguished by their ability to initiate bursts as brief sequences of spikes fired at high frequencies. Little is known about the relationship between cellular diversity and spatial coding in the subiculum. We performed high-resolution juxtacellular recordings in freely moving rats and found that bursting behavior predicts functional differences between subicular neurons. Specifically, sparsely bursting cells have lower firing rates and carry more spatial information than dominantly bursting cells. Additionally, bursts fired by sparsely bursting cells encoded spatial information better than isolated spikes, indicating that bursts act as a unit of information dedicated to spatial coding.
Keywords: border cell; cluster analysis; hippocampus; multiplexing; orientation.
Copyright © 2019 Simonnet and Brecht.
Figures


Similar articles
-
Resting and active properties of pyramidal neurons in subiculum and CA1 of rat hippocampus.J Neurophysiol. 2000 Nov;84(5):2398-408. doi: 10.1152/jn.2000.84.5.2398. J Neurophysiol. 2000. PMID: 11067982
-
Antidromic and orthodromic responses by subicular neurons in rat brain slices.Brain Res. 1997 Sep 19;769(1):71-85. doi: 10.1016/s0006-8993(97)00690-2. Brain Res. 1997. PMID: 9374275
-
Functional Diversity of Subicular Principal Cells during Hippocampal Ripples.J Neurosci. 2015 Oct 7;35(40):13608-18. doi: 10.1523/JNEUROSCI.5034-14.2015. J Neurosci. 2015. PMID: 26446215 Free PMC article.
-
Constraints on hippocampal processing imposed by the connectivity between CA1, subiculum and subicular targets.Behav Brain Res. 2006 Nov 11;174(2):265-71. doi: 10.1016/j.bbr.2006.06.014. Epub 2006 Jul 21. Behav Brain Res. 2006. PMID: 16859763 Review.
-
Two-stage model of memory trace formation: a role for "noisy" brain states.Neuroscience. 1989;31(3):551-70. doi: 10.1016/0306-4522(89)90423-5. Neuroscience. 1989. PMID: 2687720 Review.
Cited by
-
Spike Afterpotentials Shape the In Vivo Burst Activity of Principal Cells in Medial Entorhinal Cortex.J Neurosci. 2020 Jun 3;40(23):4512-4524. doi: 10.1523/JNEUROSCI.2569-19.2020. Epub 2020 Apr 24. J Neurosci. 2020. PMID: 32332120 Free PMC article.
-
Presubiculum principal cells are preserved from degeneration in knock-in APP/TAU mouse models of Alzheimer's disease.Semin Cell Dev Biol. 2023 Apr;139:55-72. doi: 10.1016/j.semcdb.2022.03.001. Epub 2022 Mar 12. Semin Cell Dev Biol. 2023. PMID: 35292192 Free PMC article.
-
Electrophysiological Characterization of Regular and Burst Firing Pyramidal Neurons of the Dorsal Subiculum in an Angelman Syndrome Mouse Model.Front Cell Neurosci. 2021 Aug 26;15:670998. doi: 10.3389/fncel.2021.670998. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34512263 Free PMC article.
-
Information Theoretic Approaches to Deciphering the Neural Code with Functional Fluorescence Imaging.eNeuro. 2021 Sep 24;8(5):ENEURO.0266-21.2021. doi: 10.1523/ENEURO.0266-21.2021. Print 2021 Sep-Oct. eNeuro. 2021. PMID: 34433574 Free PMC article.
-
Neuromodulatory functions exerted by oxytocin on different populations of hippocampal neurons in rodents.Front Cell Neurosci. 2023 Feb 2;17:1082010. doi: 10.3389/fncel.2023.1082010. eCollection 2023. Front Cell Neurosci. 2023. PMID: 36816855 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
