Cryo-electron microscopy structure of the filamentous bacteriophage IKe
- PMID: 30819888
- PMCID: PMC6431161
- DOI: 10.1073/pnas.1811929116
Cryo-electron microscopy structure of the filamentous bacteriophage IKe
Abstract
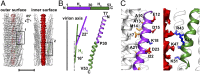
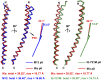
The filamentous bacteriophage IKe infects Escherichia coli cells bearing IncN pili. We report the cryo-electron microscopy structure of the micrometer-long IKe viral particle at a resolution of 3.4 Å. The major coat protein [protein 8 (p8)] consists of 47 residues that fold into a ∼68-Å-long helix. An atomic model of the coat protein was built. Five p8 helices in a horizontal layer form a pentamer, and symmetrically neighboring p8 layers form a right-handed helical cylinder having a rise per pentamer of 16.77 Å and a twist of 38.52°. The inner surface of the capsid cylinder is positively charged and has direct interactions with the encapsulated circular single-stranded DNA genome, which has an electron density consistent with an unusual left-handed helix structure. Similar to capsid structures of other filamentous viruses, strong capsid packing in the IKe particle is maintained by hydrophobic residues. Despite having a different length and large sequence differences from other filamentous phages, π-π interactions were found between Tyr9 of one p8 and Trp29 of a neighboring p8 in IKe that are similar to interactions observed in phage M13, suggesting that, despite sequence divergence, overall structural features are maintained.
Keywords: cryo-EM structure; filamentous virus; helical reconstruction; single-stranded circular DNA; symmetry mismatch.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


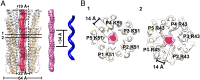
References
-
- Day LA, Marzec CJ, Reisberg SA, Casadevall A. DNA packing in filamentous bacteriophages. Annu Rev Biophys Biophys Chem. 1988;17:509–539. - PubMed
-
- Rakonjac J, Bennett NJ, Spagnuolo J, Gagic D, Russel M. Filamentous bacteriophage: Biology, phage display and nanotechnology applications. Curr Issues Mol Biol. 2011;13:51–76. - PubMed
-
- Marvin DA. X-ray diffraction and electron microscope studies on the structure of the small filamentous bacteriophage fd. J Mol Biol. 1966;15:8–17. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

