Neural crest-derived neurons invade the ovary but not the testis during mouse gonad development
- PMID: 30819894
- PMCID: PMC6431225
- DOI: 10.1073/pnas.1814930116
Neural crest-derived neurons invade the ovary but not the testis during mouse gonad development
Abstract
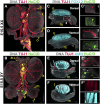
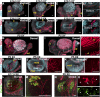
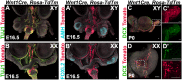
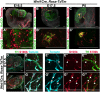
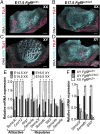
Testes and ovaries undergo sex-specific morphogenetic changes and adopt strikingly different morphologies, despite the fact that both arise from a common precursor, the bipotential gonad. Previous studies showed that recruitment of vasculature is critical for testis patterning. However, vasculature is not recruited into the early ovary. Peripheral innervation is involved in patterning development of many organs but has been given little attention in gonad development. In this study, we show that while innervation in the male reproductive complex is restricted to the epididymis and vas deferens and never invades the interior of the testis, neural crest-derived innervation invades the interior of the ovary around E16.5. Individual neural crest cells colonize the ovary, differentiate into neurons and glia, and form a dense neural network within the ovarian medulla. Using a sex-reversing mutant mouse line, we show that innervation is specific to ovary development, is not dependent on the genetic sex of gonadal or neural crest cells, and may be blocked by repressive guidance signals elevated in the male pathway. This study reveals another aspect of sexually dimorphic gonad development, establishes a precise timeline and structure of ovarian innervation, and raises many questions for future research.
Keywords: innervation; neural crest; organogenesis; ovary; testis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Characterizing the bipotential mammalian gonad.Curr Top Dev Biol. 2019;134:167-194. doi: 10.1016/bs.ctdb.2019.01.002. Epub 2019 Jan 30. Curr Top Dev Biol. 2019. PMID: 30999975 Review.
-
Dynamics of the transcriptional landscape during human fetal testis and ovary development.Hum Reprod. 2020 May 1;35(5):1099-1119. doi: 10.1093/humrep/deaa041. Hum Reprod. 2020. PMID: 32412604
-
Mutation of Gonadal soma-derived factor induces medaka XY gonads to undergo ovarian development.Biochem Biophys Res Commun. 2015 Nov 6;467(1):109-14. doi: 10.1016/j.bbrc.2015.09.112. Epub 2015 Sep 25. Biochem Biophys Res Commun. 2015. PMID: 26408909
-
Development of Xenopus laevis bipotential gonads into testis or ovary is driven by sex-specific cell-cell interactions, proliferation rate, cell migration and deposition of extracellular matrix.Dev Biol. 2017 Dec 15;432(2):298-310. doi: 10.1016/j.ydbio.2017.10.020. Epub 2017 Nov 7. Dev Biol. 2017. PMID: 29080791
-
Mammalian sex determination and gonad development.Curr Top Dev Biol. 2013;106:89-121. doi: 10.1016/B978-0-12-416021-7.00003-1. Curr Top Dev Biol. 2013. PMID: 24290348 Review.
Cited by
-
A PITX2-HTR1B pathway regulates the asymmetric development of female gonads in chickens.PNAS Nexus. 2023 Jun 19;2(6):pgad202. doi: 10.1093/pnasnexus/pgad202. eCollection 2023 Jun. PNAS Nexus. 2023. PMID: 37388922 Free PMC article.
-
Innervation in organogenesis.Curr Top Dev Biol. 2022;148:195-235. doi: 10.1016/bs.ctdb.2022.02.004. Epub 2022 Mar 12. Curr Top Dev Biol. 2022. PMID: 35461566 Free PMC article.
-
Intrinsic innervation of the ovary and its variations in the rat senescence process.J Mol Histol. 2022 Apr;53(2):347-356. doi: 10.1007/s10735-022-10069-7. Epub 2022 Feb 25. J Mol Histol. 2022. PMID: 35217964 Free PMC article.
-
Single-cell transcriptomic profiling of the neonatal oviduct and uterus reveals new insights into upper Müllerian duct regionalization.FASEB J. 2024 May 15;38(9):e23632. doi: 10.1096/fj.202400303R. FASEB J. 2024. PMID: 38686936 Free PMC article.
-
Mesenchymal stem cells derived from hPSC via neural crest attenuate chemotherapy-induced premature ovarian insufficiency by ameliorating apoptosis and oxidative stress in granulosa cells.Stem Cell Res Ther. 2025 May 13;16(1):239. doi: 10.1186/s13287-025-04346-x. Stem Cell Res Ther. 2025. PMID: 40361250 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

