Dynamic prediction of bleeding risk in thrombocytopenic preterm neonates
- PMID: 30819913
- PMCID: PMC6821634
- DOI: 10.3324/haematol.2018.208595
Dynamic prediction of bleeding risk in thrombocytopenic preterm neonates
Abstract
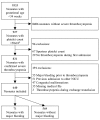
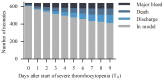
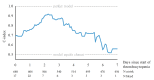
Over 75% of severely thrombocytopenic neonates receive platelet transfusions, though little evidence supports this practice, and only 10% develop major bleeding. In a recent randomized trial, giving platelet transfusions at a threshold platelet count of 50x109/L compared to a threshold of 25x109/L was associated with an increased risk of major bleeding or mortality. This finding highlights the need for improved and individualized guidelines on neonatal platelet transfusion, which require accurate prediction of bleeding risk. Therefore, the objective of this study was to develop a dynamic prediction model for major bleeding in thrombocytopenic preterm neonates. This model allows for calculation of bleeding risk at any time-point during the first week after the onset of severe thrombocytopenia. In this multicenter cohort study, we included neonates with a gestational age <34 weeks, admitted to a neonatal intensive care unit, who developed severe thrombocytopenia (platelet count <50x109/L). The study endpoint was major bleeding. We obtained predictions of bleeding risk using a proportional baselines landmark supermodel. Of 640 included neonates, 71 (11%) had a major bleed. We included the variables gestational age, postnatal age, intrauterine growth retardation, necrotizing enterocolitis, sepsis, platelet count and mechanical ventilation in the model. The median cross-validated c-index was 0.74 (interquartile range, 0.69-0.82). This is a promising dynamic prediction model for bleeding in this population that should be explored further in clinical studies as a potential instrument for supporting clinical decisions. The study was registered at www.clinicaltrials.gov (NCT03110887).
Copyright© 2019 Ferrata Storti Foundation.
Figures

References
-
- Baer VL, Lambert DK, Henry E, Christensen RD. Severe thrombocytopenia in the NICU. Pediatrics. 2009;124(6):e1095–100. - PubMed
-
- Fustolo-Gunnink SF, Huijssen-Huisman EJ, van der Bom JG, et al. Are thrombocytopenia and platelet transfusions associated with major bleeding in preterm neonates? A sys tematic review. Blood Rev. 2019;36:1–9. - PubMed
-
- Stanworth SJ, Clarke P, Watts T, et al. Prospective, observational study of outcomes in neonates with severe thrombocytopenia. Pediatrics. 2009;124(5):e826–34. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

