Accidental intoxications in toddlers: lack of cross-reactivity of vilazodone and its urinary metabolite M17 with drug of abuse screening immunoassays
- PMID: 30820187
- PMCID: PMC6379996
- DOI: 10.1186/s12907-019-0084-9
Accidental intoxications in toddlers: lack of cross-reactivity of vilazodone and its urinary metabolite M17 with drug of abuse screening immunoassays
Abstract
Background: Vilazodone is an FDA approved medication used to treat major depressive disorder. The authors describe two cases of accidental vilazodone exposure in toddlers who presented with symptoms similar to amphetamine exposure and also with unexplained positive amphetamine urine immunoassay drug screens. Given a lack of published data on cross-reactivity of vilazodone and its metabolites with drug of abuse screening tests, the authors investigated drug of abuse immunoassay cross-reactivity of vilazodone and metabolites using computational and empirical approaches.
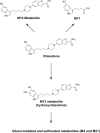
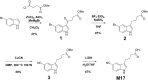
Methods: To ascertain the likelihood that vilazodone would cross-react with drug of abuse screening immunoassays, the authors assessed the two-dimensional (2D) similarity of the vilazodone parent molecule and known metabolites to an array of antigenic targets for urine immunoassay drug screens. To facilitate studies of the commercially unavailable M17 metabolite, it was prepared synthetically through a novel scheme. Urine and serum were spiked with vilazodone and M17 into urine (200-100,000 ng/mL) and serum (20-2000 ng/mL) samples and tested for cross-reactivity.
Results: Computational analysis using 2D similarity showed that vilazodone and metabolites have generally low similarity to antigenic targets of common drug of abuse screening immunoassays, predicting weak or no cross-reactivity. The M17 metabolite had 2D similarity to amphetamines and tricyclic antidepressants in a range similar to some other compounds exhibiting weak cross-reactivity on these immunoassays. Cross-reactivity testing was therefore performed on two different urine amphetamines immunoassays and a serum tricyclic antidepressant immunoassay. However, actual testing of cross reactivity for vilazodone and the M17 metabolite did not detect cross-reactivity for any urine amphetamines screen at concentrations up to 100,000 ng/mL and for a serum tricyclic antidepressants assays at concentrations up to 2000 ng/mL.
Conclusion: While the vilazodone metabolite M17 has weak 2D structural similarity to amphetamines and tricyclic antidepressants, the current study did not demonstrate any experimental cross-reactivity with two different urine amphetamines immunoassays and a serum tricyclic antidepressant immunoassay. Vilazodone ingestions in young children present a diagnostic challenge in their similarity to amphetamine ingestions and the lack of routine laboratory tests for vilazodone. Further work is needed to understand the metabolic profile for vilazodone in children versus adults.
Keywords: Amphetamines; False positive reactions; Immunoassay; Similarity; Toxicology.
Conflict of interest statement
This study had approval from the University of Iowa Institutional Review Board as a retrospective study (protocol #201705809).Not applicable.The authors all declare no conflicts of interest apart from SE who is the Chief Executive Officers of Collaborations Pharmaceuticals, Inc.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Using molecular similarity to highlight the challenges of routine immunoassay-based drug of abuse/toxicology screening in emergency medicine.BMC Emerg Med. 2009 Apr 28;9:5. doi: 10.1186/1471-227X-9-5. BMC Emerg Med. 2009. PMID: 19400959 Free PMC article.
-
Cross-reactivity studies and predictive modeling of "Bath Salts" and other amphetamine-type stimulants with amphetamine screening immunoassays.Clin Toxicol (Phila). 2013 Feb;51(2):83-91. doi: 10.3109/15563650.2013.768344. Clin Toxicol (Phila). 2013. PMID: 23387345
-
False positive amphetamines and 3,4-methylenedioxymethamphetamine immunoassays in the presence of metoprolol-two cases reported in clinical toxicology.J Anal Toxicol. 2020 Mar 7;44(2):200-205. doi: 10.1093/jat/bkz051. J Anal Toxicol. 2020. PMID: 31384953
-
Cross-reactivity of commercial immunoassays for screening of new amphetamine designer drugs. A review.J Pharm Biomed Anal. 2022 Sep 5;218:114868. doi: 10.1016/j.jpba.2022.114868. Epub 2022 Jun 3. J Pharm Biomed Anal. 2022. PMID: 35688007 Review.
-
Psychiatric and non-psychiatric drugs causing false-positive amphetamines urine test in psychiatric patients: a pharmacovigilance analysis using FAERS.Expert Rev Clin Pharmacol. 2023 May;16(5):453-465. doi: 10.1080/17512433.2023.2211261. Epub 2023 May 15. Expert Rev Clin Pharmacol. 2023. PMID: 37147189 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources