N, N-dimethylformamide induces cellulase production in the filamentous fungus Trichoderma reesei
- PMID: 30820246
- PMCID: PMC6380019
- DOI: 10.1186/s13068-019-1375-1
N, N-dimethylformamide induces cellulase production in the filamentous fungus Trichoderma reesei
Abstract
Background: The filamentous fungus Trichoderma reesei produces cellulase enzymes that are widely studied for lignocellulose bioconversion to biofuel. N,N-dimethylformamide (DMF) is a versatile organic solvent used in large quantities in industries.
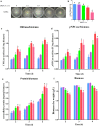
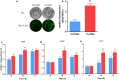
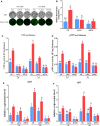
Results: In this study, we serendipitously found that biologically relevant concentrations of extracellular DMF-induced cellulase production in the T. reesei hyper-cellulolytic mutant Rut-C30 and wild-type strain QM6a. Next, by transcriptome analysis, we determined that plc-e encoding phospholipase C was activated by DMF and revealed that cytosolic Ca2+ plays a vital role in the response of T. reesei to DMF. Using EGTA (a putative extracellular Ca2+ chelator) and LaCl3 (a plasma membrane Ca2+ channel blocker), we demonstrated that DMF induced a cytosolic Ca2+ burst via extracellular Ca2+ and Ca2+ channels in T. reesei, and that the cytosolic Ca2+ burst induced by DMF-mediated overexpression of cellulase through calcium signaling. Deletion of crz1 confirmed that calcium signaling plays a dominant role in DMF-induced cellulase production. Additionally, 0.5-2% DMF increases the permeability of T. reesei mycelia for cellulase release. Simultaneous supplementation with 1% DMF and 10 mM Mn2+ to T. reesei Rut-C30 increased cellulase activity approximately fourfold compared to that without treatment and was also more than that observed in response to either treatment alone.
Conclusions: Our results reveal that DMF-induced cellulase production via calcium signaling and permeabilization. Our results also provide insight into the role of calcium signaling in enzyme production for enhanced cellulase production and the development of novel inducers of cellulase.
Keywords: Calcium signaling; Cellulase; DMF induced; Permeability; Phospholipase C; Trichoderma reesei; plc-e.
Figures




Similar articles
-
Mechanism of Zn2+ regulation of cellulase production in Trichoderma reesei Rut-C30.Biotechnol Biofuels Bioprod. 2023 Apr 28;16(1):73. doi: 10.1186/s13068-023-02323-1. Biotechnol Biofuels Bioprod. 2023. PMID: 37118821 Free PMC article.
-
cAMP activates calcium signalling via phospholipase C to regulate cellulase production in the filamentous fungus Trichoderma reesei.Biotechnol Biofuels. 2021 Mar 8;14(1):62. doi: 10.1186/s13068-021-01914-0. Biotechnol Biofuels. 2021. PMID: 33685506 Free PMC article.
-
Mn2+ modulates the expression of cellulase genes in Trichoderma reesei Rut-C30 via calcium signaling.Biotechnol Biofuels. 2018 Mar 1;11:54. doi: 10.1186/s13068-018-1055-6. eCollection 2018. Biotechnol Biofuels. 2018. PMID: 29507606 Free PMC article.
-
Deciphering the molecular mechanisms behind cellulase production in Trichoderma reesei, the hyper-cellulolytic filamentous fungus.Biosci Biotechnol Biochem. 2016 Sep;80(9):1712-29. doi: 10.1080/09168451.2016.1171701. Epub 2016 Apr 14. Biosci Biotechnol Biochem. 2016. PMID: 27075508 Review.
-
Trichoderma reesei RUT-C30--thirty years of strain improvement.Microbiology (Reading). 2012 Jan;158(Pt 1):58-68. doi: 10.1099/mic.0.054031-0. Epub 2011 Oct 13. Microbiology (Reading). 2012. PMID: 21998163 Review.
Cited by
-
Enhancing cellulase biosynthesis of Bacillus subtilis Z2 by regulating intracellular NADH level.iScience. 2025 Apr 3;28(5):112341. doi: 10.1016/j.isci.2025.112341. eCollection 2025 May 16. iScience. 2025. PMID: 40276757 Free PMC article.
-
Optimizing the amount of pig manure in the vermicomposting of spent mushroom (Lentinula) substrate.PeerJ. 2020 Dec 18;8:e10584. doi: 10.7717/peerj.10584. eCollection 2020. PeerJ. 2020. PMID: 33384904 Free PMC article.
-
The CBS/H2S signalling pathway regulated by the carbon repressor CreA promotes cellulose utilization in Ganoderma lucidum.Commun Biol. 2024 Apr 17;7(1):466. doi: 10.1038/s42003-024-06180-y. Commun Biol. 2024. PMID: 38632386 Free PMC article.
-
Regulatory Role of Vacuolar Calcium Transport Proteins in Growth, Calcium Signaling, and Cellulase Production in Trichoderma reesei.J Fungi (Basel). 2024 Dec 11;10(12):853. doi: 10.3390/jof10120853. J Fungi (Basel). 2024. PMID: 39728349 Free PMC article.
-
Mechanism of Zn2+ regulation of cellulase production in Trichoderma reesei Rut-C30.Biotechnol Biofuels Bioprod. 2023 Apr 28;16(1):73. doi: 10.1186/s13068-023-02323-1. Biotechnol Biofuels Bioprod. 2023. PMID: 37118821 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

