Use of amantadine as substrate for SSAT-1 activity as a reliable clinical diagnostic assay for breast and lung cancer
- PMID: 30820345
- PMCID: PMC6391627
- DOI: 10.4155/fsoa-2018-0106
Use of amantadine as substrate for SSAT-1 activity as a reliable clinical diagnostic assay for breast and lung cancer
Abstract
Aim: Spermidine/spermine N1-acetyltransferase (SSAT-1) plays a critical role in cell growth, proliferation and death, and is known to be activated in human cancer cells. Amantadine, a US FDA-approved antiviral drug, is a substrate for SSAT-1 and can be used to indirectly measure SSAT-1 activity because of its conversion to acetylamantadine (AA). This study was undertaken to further validate SSAT-1 activity in breast and lung cancer patients.
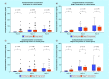
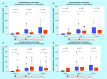
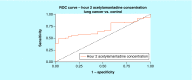
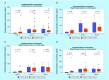
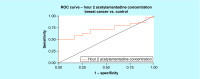
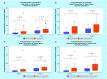
Results: An increase in the urinary concentration of AA in lung and breast cancer patients was observed. The 0-2 h collection time point was determined to be optimal in revealing significant differences in urinary AA concentration between healthy controls and cancer patients.
Conclusion: The high urine concentration of AA could be used as a simple and useful test for the detection of breast and lung cancer.
Keywords: SSAT-1; amantadine; biomarkers; breast cancer; cancer diagnostics and screening; lung cancer; polyamine metabolism.
Conflict of interest statement
Financial & competing interests disclosure This study was supported, in part, by Biomark Diagnostics, Inc. (Richmond, BC, Canada) and the Maunders-McNeil Foundation (Edmonton, AB, Canada). The study was also funded by the University of Manitoba and Biomark Diagnostics, Inc. None of the authors has conflict of interest to disclose. However, RB Ahmed is the President and CEO and B Cheng is the acting CTO of BioMark Diagnostics, Inc. A Maksymiuk, DS Sitar, H Bach, PS Tappia and B Ramjiawan are minor shareholders of BioMark Diagnostics, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Figures

References
-
- Global cancer facts & figures. 2018. www.cancer.org/research/cancer-facts-statistics/global.html
-
- International Agency for Cancer Research. 2018. http://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports...
-
- Foreman KJ, Marquez N, Dolgert A, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet. 2018 doi: 10.1016/S0140(18)31694-5. - DOI - PMC - PubMed
-
•• An outstanding article that describes a novel approach to modeling life expectancy, all-cause mortality and cause of death forecasts for 250 causes of death from 2016 to 2040 in 195 countries and territories.
-
- World Cancer Research Fund International, Breast Cancer Statistics. 2017. www.wcrf.org/int/cancer-facts-figures/data-specific-cancers/breast-cance...
-
- American Cancer Society. American Cancer Society; Atlanta, GA, USA: 2017. Cancer Facts and Figures 2017.
LinkOut - more resources
Full Text Sources
Research Materials
