Tacrolimus Suppositories in Therapy-Resistant Ulcerative Proctitis
- PMID: 30820433
- PMCID: PMC6390495
- DOI: 10.1159/000493979
Tacrolimus Suppositories in Therapy-Resistant Ulcerative Proctitis
Abstract
Background: Ulcerative proctitis may often be managed with topical salicylates or steroids alone, but in some patients, symptoms are persistent and severe. We analyzed the efficacy of tacrolimus suppositories in patients who had proven refractory to combined topical and systemic treatment.
Methods: In this retrospective analysis, ulcerative colitis activity index (CAI), side effects, co-medication and drug levels were assessed in 43 patients with distal ulcerative colitis who received suppositories containing 2 mg of tacrolimus b.i.d. as add-on medication.
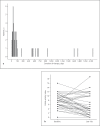
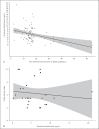
Results: A total of 23 patients with ulcerative proctitis presented to follow-up within ≤50 days (mean 27.0 days) after suppositories were started. A decrease in CAI (from 8.0 to 5.5 points) was observed and 52.3% reached clinical remission (CAI ≤4). In total, 43 patients were available for analysis, of whom 9 had inflammation of the sigmoid colon as well. For the entire cohort, the median treatment duration was 76 days; 60% were in remission on the last documented visit. Serum measurements revealed a substantial tacrolimus level with a mean of 5.5 ng/mL. We observed one case of mild reversible acute kidney injury.
Conclusions: In ulcerative proctitis, adding tacrolimus suppositories can be an effective and safe option when topical mesalazine, corticoid formulations and concomitant oral or parenteral medications have failed.
Keywords: Inflammatory bowel disease; Tacrolimus; Therapy resistant; Topical treatment; Ulcerative proctitis.
Figures

References
-
- Dignass A, Preiss JC, Aust DE, Autschbach F, Ballauff A, Barretton G, et al. Aktualisierte Leitlinie zur Diagnostik und Therapie der Colitis ulcerosa 2011 - Ergebnisse einer Evidenzbasierten Konsensuskonferenz. Z Gastroenterol. 2011 Sep;49((9)):1276–341. - PubMed
-
- Klag T, Mazurak N, Fantasia L, Schwille-Kiuntke J, Kirschniak A, Falch C, et al. High Demand for Psychotherapy in Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017 Oct;23((10)):1796–802. - PubMed
-
- Escher M, Stange EF, Herrlinger KR. Two cases of fatal Pneumocystis jirovecii pneumonia as a complication of tacrolimus therapy in ulcerative colitis—a need for prophylaxis. J Crohn's Colitis. 2010 Nov;4((5)):606–9. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

