Vascular RAGE transports oxytocin into the brain to elicit its maternal bonding behaviour in mice
- PMID: 30820471
- PMCID: PMC6389896
- DOI: 10.1038/s42003-019-0325-6
Vascular RAGE transports oxytocin into the brain to elicit its maternal bonding behaviour in mice
Abstract
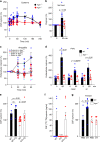
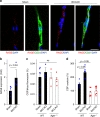
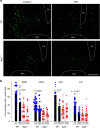
Oxytocin sets the stage for childbirth by initiating uterine contractions, lactation and maternal bonding behaviours. Mice lacking secreted oxcytocin (Oxt -/-, Cd38 -/-) or its receptor (Oxtr -/-) fail to nurture. Normal maternal behaviour is restored by peripheral oxcytocin replacement in Oxt -/- and Cd38 -/-, but not Oxtr -/- mice, implying that circulating oxcytocin crosses the blood-brain barrier. Exogenous oxcytocin also has behavioural effects in humans. However, circulating polypeptides are typically excluded from the brain. We show that oxcytocin is transported into the brain by receptor for advanced glycation end-products (RAGE) on brain capillary endothelial cells. The increases in oxcytocin in the brain which follow exogenous administration are lost in Ager -/- male mice lacking RAGE, and behaviours characteristic to abnormalities in oxcytocin signalling are recapitulated in Ager -/- mice, including deficits in maternal bonding and hyperactivity. Our findings show that RAGE-mediated transport is critical to the behavioural actions of oxcytocin associated with parenting and social bonding.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Carter, C. S. Oxytocin and human evolution. Curr. Top. Behav. Neurosci. 35, 291–319 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

