Soluble Urokinase-Type Plasminogen Activator Receptor and Arterial Stiffness in Patients with COPD
- PMID: 30820636
- PMCID: PMC6486892
- DOI: 10.1007/s00408-019-00211-w
Soluble Urokinase-Type Plasminogen Activator Receptor and Arterial Stiffness in Patients with COPD
Abstract
Introduction: Soluble urokinase-type plasminogen activator receptor (suPAR) is upregulated by inflammation and plays a role in the pathogenesis of atherosclerosis. Chronic obstructive pulmonary disease (COPD) is associated with enhanced systemic inflammation and increased risk for atherosclerosis, however, studies analysing the circulating suPAR levels in COPD are contradictory. The aim of the study was to investigate plasma suPAR concentrations together with markers of arterial stiffness in COPD.
Materials and methods: Twenty-four patients with COPD and 18 non-COPD, control subjects participated in the study. Plasma suPAR was measured, together with lung volumes, symptom burden, exacerbation history, markers of arterial stiffness and soluble inflammatory biomarkers, such as endothelin-1, high-sensitivity C-reactive protein (hsCRP), interleukin-6 (IL-6).
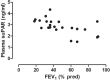
Results: Plasma suPAR levels were higher in COPD (2.84 ± 0.67 ng/ml vs. 2.41 ± 0.57 ng/ml, p = 0.03) and were related to lung function measured with FEV1 (r = - 0.65, p < 0.01) and symptom burden determined with the modified Medical Research Council questionnaire (r = 0.55, p < 0.05). Plasma suPAR concentrations correlated with various measures of arterial stiffness in all subjects, but only with ejection duration in COPD (r = - 0.44, p = 0.03).
Conclusions: Plasma suPAR levels are elevated in COPD and relate to arterial stiffness. Our results suggest that suPAR may be a potential link between COPD and atherosclerosis.
Keywords: Arterial stiffness; COPD; Cardiovascular risk; IL-6; SuPAR.
Conflict of interest statement
Conflict of interest
Miklós Illyés is a patent owner of the Arteriograph method and has shares in TensioMed Ltd., a company that manufactures the Arteriograph device for measuring arterial stiffness. The other authors declare no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Figures



References
-
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 Report: GOLD executive summary. Eur Respir J. 2017 - PubMed
-
- Coxson HO, Dirksen A, Edwards LD, Evaluation of. CLtIPSEI et al. The presence and progression of emphysema in COPD as determined by CT scanning and biomarker expression: a prospective analysis from the ECLIPSE study. Lancet Respir Med. 2013;1(2):129–136. doi: 10.1016/S2213-2600(13)70006-7. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

