IgE Glycosylation in Health and Disease
- PMID: 30820668
- PMCID: PMC6750212
- DOI: 10.1007/82_2019_151
IgE Glycosylation in Health and Disease
Abstract
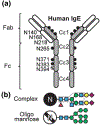
IgE are absolutely required for initiation of allergy reactions, which affect over 20% of the world's population. IgE are the least prevalent immunoglobulins in circulation with 12-h and 2-day half-lives in mouse and human serum, respectively, but an extended tissue half-life of 3-weeks bound to the surface of mast cells by the high affinity IgE receptor, FcεRI (Gould and Sutton 2008). Although the importance of glycosylation to IgG biology is well established, less is known regarding the contribution of IgE glycosylation to allergic inflammation. IgE has seven and nine N-linked glycosylation sites distributed across human and murine constant chains, respectively. Here we discuss studies that have analyzed IgE glycosylation and its function, and how IgE glycosylation contributions to health and disease.
Figures
References
-
- 2010 Asthma’s Impact on the Nation; Data from the CDC National Asthma Control Program. Center for Disease Control
-
- Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ, Dugast AS, Heizen EL, Ercan A, Choi I, Streeck H, Nigrovic PA, Bailey-Kellogg C, Scanlan C, Alter G (2013a) Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest 123(5):2183–2192. 10.1172/JCI65708 - DOI - PMC - PubMed
-
- Ackerman ME, Dugast AS, McAndrew EG, Tsoukas S, Licht AF, Irvine DJ, Alter G (2013b) Enhanced phagocytic activity of HIV-specific antibodies correlates with natural production of immunoglobulins with skewed affinity for FcγR2a and FcγR2b. J Virol 87(10):5468–5476. 10.1128/JVI.03403-12 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

