Ocrelizumab efficacy in subgroups of patients with relapsing multiple sclerosis
- PMID: 30820738
- PMCID: PMC6469695
- DOI: 10.1007/s00415-019-09248-6
Ocrelizumab efficacy in subgroups of patients with relapsing multiple sclerosis
Abstract
Objective: The efficacy and safety of ocrelizumab, versus interferon (IFN) β-1a, for the treatment of relapsing multiple sclerosis (RMS) from the identically designed OPERA I (NCT01247324) and OPERA II (NCT01412333) phase III studies has been reported; here we present subgroup analyses of efficacy endpoints from the pooled OPERA I and OPERA II populations.
Methods: Patients with RMS were randomized to either ocrelizumab 600 mg administered by intravenous infusion every 24 weeks or subcutaneous IFN β-1a 44 µg three times per week throughout the 96-week treatment period. Relapse, disability, and MRI outcomes were analyzed for predefined and post hoc subgroups based on demographic and disease characteristics along with prior treatment using appropriate statistical tests to determine the treatment effect in subgroups and treatment-by-subgroup interactions.
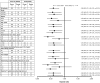
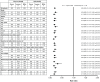
Results: The significant treatment benefit of ocrelizumab, versus IFN β-1a, observed in the overall OPERA I and OPERA II pooled populations was maintained across most subgroup strata for all endpoints, including annualized relapse rate, disability progression, and MRI outputs.
Conclusions: The treatment effect of ocrelizumab versus IFN β-1a, measured by clinical and MRI outcomes, was maintained across most of the subgroups and strata of interest, and the pattern of treatment benefit across all subgroups was consistent with that from the pooled OPERA studies.
Keywords: Interferon β-1a; Multiple sclerosis; Ocrelizumab; Phase 3; Relapsing; Subgroup.
Conflict of interest statement
Conflicts of interest
B. Turner has received honoraria, travel grants and been a member of advisory boards for Biogen, Merck Serono, Novartis, Sanofi Genzyme, and Roche. C. Papeix has received speaking honoraria and travel expense reimbursement for participation in scientific meetings, and has participated in advisory boards in the past years with Biogen, MedDay, Merck Serono, Novartis, Roche, and Sanofi Genzyme. B. Cree has received personal compensation for consulting from AbbVie, Akili, Biogen, EMD Serono, GeNeuro, Novartis, and Sanofi Genzyme. L. Kappos’s institution, the University Hospital Basel, has received research support and payments that were used exclusively for research support for Prof. Kappos’s activities as principal investigator and member or chair of planning and steering committees or advisory boards in trials sponsored by Actelion, Addex, Almirall, Bayer HealthCare Pharmaceuticals, CSL Behring, Genentech, Inc., GeNeuro SA, Genzyme, Merck Serono, Mitsubishi Pharma, Novartis, Octapharma, Ono Pharma, Pfizer, Receptos, F. Hoffmann-La Roche Ltd, Sanofi, Santhera, Siemens, Teva, UCB, and XenoPort; license fees for Neurostatus products; and research grants from the Swiss MS Society, the Swiss National Research Foundation, the European Union, the Gianni Rubatto Foundation, the Novartis Research Foundation and the Roche Research Foundation. X. Montalban has received speaking honoraria and travel expense reimbursement for participation in scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past years with Actelion, Almirall, Bayer, Biogen, Genzyme, Merck, Novartis, Octapharma, Receptos, F. Hoffmann-La Roche Ltd, Sanofi, Teva, and Trophos. J.S. Wolinsky has served on advisory boards, data monitoring or steering committees, has consulting agreements, or received speaker honoraria from the following entities: AbbVie, AcademicCME, Actelion, ACTRIMS, Alkermes, Bayer HealthCare, Biogen, Bionest, Celgene, Clene Nanomedicine, CMSC, ECTRIMS, EMS Serono, Forward Pharma A/S, France Foundation, GeNeuro, MA’s Communications, Masters MS, MedDay Pharmaceuticals, McDonnell Boehnen Hulbert & Berghoff, Novartis Pharmaceuticals, Otsuka, PlatformQ Health Education, PRIME, PTC Therapeutics, Roche Genentech, Sanofi Genzyme, Strategic Consultants International, Takeda, Teva Pharmaceuticals, and WebMD; royalties are received for out-licensed monoclonal antibodies through UTHealth from Millipore Corporation. R. Buffels is an employee of F. Hoffmann-La Roche Ltd. H. Garren is an employee of Genentech, Inc., and a shareholder of F. Hoffmann-La Roche Ltd. D. Fiore is an employee of Genentech, Inc., and a shareholder of F. Hoffmann-La Roche Ltd. J. Han is an employee of Genentech, Inc. S.L. Hauser serves on the board of trustees for Neurona and on scientific advisory boards for Annexon, Symbiotix, Bionure, Alector, and Molecular Stethoscope; he has also received travel reimbursement from F. Hoffmann-La Roche Ltd for CD20-related meetings and presentations.
Ethical standards
This trial was conducted in accordance with the International Conference on Harmonisation guidelines for Good Clinical Practice and the Declaration of Helsinki.
Informed consent
All patients provided written informed consent.
Figures




References
-
- Papeix C, Cree B, Turner B et al (2017) Subgroup analyses of annualised relapse rates in patients with relapsing multiple sclerosis who received ocrelizumab or interferon beta-1a in the phase III OPERA I and OPERA II studies. In: 7th Joint European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Meeting; 25–28 October 2017; Paris, France. Poster P687

