Physical exercise training for type 3 spinal muscular atrophy
- PMID: 30821348
- PMCID: PMC6396106
- DOI: 10.1002/14651858.CD012120.pub2
Physical exercise training for type 3 spinal muscular atrophy
Abstract
Background: Physical exercise training might improve muscle and cardiorespiratory function in spinal muscular atrophy (SMA). Optimization of aerobic capacity or other resources in residual muscle tissue through exercise may counteract the muscle deterioration that occurs secondary to motor neuron loss and inactivity in SMA. There is currently no evidence synthesis available on physical exercise training in people with SMA type 3.
Objectives: To assess the effects of physical exercise training on functional performance in people with SMA type 3, and to identify any adverse effects.
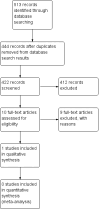
Search methods: On 8 May 2018, we searched the Cochrane Neuromuscular Specialised Register, Cochrane Central Register of Controlled Trials, MEDLINE, Embase, CINAHL, AMED, and LILACS. On 25 April 2018 we searched NHSEED, DARE, and ClinicalTrials.gov and WHO ICTRP for ongoing trials.
Selection criteria: We included randomized controlled trials (RCTs) or quasi-RCTs lasting at least 12 weeks that compared physical exercise training (strength training, aerobic exercise training, or both) to placebo, standard or usual care, or another type of non-physical intervention for SMA type 3. Participants were adults and children from the age of five years with a diagnosis of SMA type 3 (Kugelberg-Welander syndrome), confirmed by genetic analysis.
Data collection and analysis: We used standard Cochrane methodological procedures.
Main results: We included one RCT that studied the effects of a six-month, home-based, combined muscle strength and recumbent cycle ergometry training program versus usual care in 14 ambulatory people with SMA. The age range of the participants was between 10 years and 48 years. The study was evaluator-blinded, but personnel and participants could not be blinded to the intervention, which placed the results at a high risk of bias. Participants performed strength training as prescribed, but 50% of the participants did not achieve the intended aerobic exercise training regimen. The trial used change in walking distance on the six-minute walk test as a measure of function; a minimal detectable change is 24.0 m. The change from baseline to six months' follow-up in the training group (9.4 m) was not detectably different from the change in the usual care group (-0.14 m) (mean difference (MD) 9.54 m, 95% confidence interval (CI) -83.04 to 102.12; N = 12). Cardiopulmonary exercise capacity, assessed by the change from baseline to six months' follow-up in peak oxygen uptake (VO2max) was similar in the training group (-0.12 mL/kg/min) and the usual care group (-1.34 mL/kg/min) (MD 1.22 mL/kg/min, 95% CI -2.16 to 4.6; N = 12). A clinically meaningful increase in VO2max is 3.5 mL/kg/min.The trial assessed function on the Hammersmith Functional Motor Scale - Expanded (HFMSE), which has a range of possible scores from 0 to 66, with an increase of 3 or more points indicating clinically meaningful improvement. The HFMSE score in the training group increased by 2 points from baseline to six months' follow-up, with no change in the usual care group (MD 2.00, 95% CI -2.06 to 6.06; N = 12). The training group showed a slight improvement in muscle strength, expressed as the manual muscle testing (MMT) total score, which ranges from 28 (weakest) to 280 (strongest). The change from baseline in MMT total score was 6.8 in the training group compared to -5.14 in the usual care group (MD 11.94, 95% CI -3.44 to 27.32; N = 12).The trial stated that training had no statistically significant effects on fatigue and quality of life. The certainty of evidence for all outcomes was very low because of study limitations and imprecision. The study did not assess the effects of physical exercise training on physical activity levels. No study-related serious adverse events or adverse events leading to withdrawal occurred, but we cannot draw wider conclusions from this very low-certainty evidence.
Authors' conclusions: It is uncertain whether combined strength and aerobic exercise training is beneficial or harmful in people with SMA type 3, as the quality of evidence is very low. We need well-designed and adequately powered studies using protocols that meet international standards for the development of training interventions, in order to improve our understanding of the exercise response in people with SMA type 3 and eventually develop exercise guidelines for this condition.
Conflict of interest statement
BB: no known conflicts of interest.
WLvP: obtained research grants from Prinses Beatrix Spierfonds and Stichting Spieren voor Spieren, both non‐profit foundations. His employer receives fees for SMA‐related (ad hoc) consultancy activities.
JFdG: no known conflicts of interest.
JM: was an investigator in the completed trial on the effect of exercise training in ambulatory patients with SMA (Montes 2015). The United States Department of Defense funded the trial. However, since the completion of the trial, JM has not received funding for the study (NCT01166022). JM receives research support from Eunice Kennedy Shriver Institute for Child Health and Human Development (NICHD) and the Muscular Dystrophy Association (MDA) and has no other conflicts of interest.
Figures




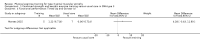









Comment in
-
Does physical exercise training improve functional performance in type 3 spinal muscular atrophy? A Cochrane Review summary with commentary.Dev Med Child Neurol. 2020 Sep;62(9):1014-1016. doi: 10.1111/dmcn.14622. Epub 2020 Jul 4. Dev Med Child Neurol. 2020. PMID: 32621614 No abstract available.
References
References to studies included in this review
Montes 2015 {published and unpublished data}
-
- B. Bartels. Allocation concealment used in our trial. Email to: Montes 2 August 2017.
-
- Montes J, Garber CE, Kramer SS, Montgomery MJ, Dunaway S, Kamil‐Rosenberg S, et al. A randomized, controlled clinical trial of exercise in patients with spinal muscular atrophy: methods and baseline characteristics. Journal of Neuromuscular Diseases 2014;1(2):151‐61. [PUBMED: 27858768] - PubMed
-
- Montes J, Garber CE, Kramer SS, Montgomery MJ, Dunaway S, Kamil‐Rosenberg S, et al. Single‐blind, randomized, controlled clinical trial of exercise in ambulatory spinal muscular atrophy: why are the results negative?. Journal of Neuromuscular Diseases 2015;2(4):463‐70. [PUBMED: 27858749] - PMC - PubMed
References to studies excluded from this review
Basoglu 2006 {published data only}
-
- Basoglu B, Karaduman A, Ozgen A. Spinal muskuler atrofili olgularda ev programinin kas kuvveti ve motor fonksiyon uzerine etkileri [Spinal muskuler atrofili olgularda ev programinin kas kuvveti ve motor fonksiyon uzerine etkileri]. Fizyoterapi Rehabilitasyon 2006;17(1):3‐9.
Cunha 1996 {published data only}
-
- Cunha MCB, Oliveira ASB, Labronici RHDD, Gabbai AA. Spinal muscular atrophy type 2 (intermediary) and 3 (Kugelberg‐Welander). Evolution of 50 patients with physiotherapy and hydrotherapy in a swimming pool. Arquivos de Neuro‐psiquiatria 1996;54(3):402‐6. - PubMed
Dahl 2004 {published data only}
-
- Dahl A, Skjeidal OH, Simensen A, Dalen HE, Bråthen T, Ahlvin P, et al. Treatment of patients with neuromuscular disease in a warm climate [Behandling i varmt klima for pasienter med nevromuskulaere sykdommer]. Tidsskr Nor Lægeforen 2004; Vol. 13–14, issue 124:1795–8. [PUBMED: 15229669] - PubMed
Lewelt 2015 {published data only}
Madsen 2015 {published data only}
-
- Madsen KL, Hansen RS, Preisler N, Thogersen F, Berthelsen MP, Vissing J. Training improves oxidative capacity, but not function, in spinal muscular atrophy type III. Muscle & Nerve 2015;52(2):240‐4. [PUBMED: 25418505] - PubMed
McCartney 1988 {published data only}
-
- McCartney N, Moroz D, Garner SH, McComas AJ. The effects of strength training in patients with selected neuromuscular disorders. Medicine and Science in Sports and Exercise 1988;20(4):362‐8. [PUBMED: 3173043] - PubMed
Milner‐Brown 1988 {published data only}
-
- Milner‐Brown HS, Miller RG. Muscle strengthening through high resistance weight training in patients with neuromuscular disorders. Archives of Physical Medicine and Rehabilitation 1988;69(1):14‐9. [PUBMED: 3337636] - PubMed
Salem 2010 {published data only}
-
- Salem Y, Gropack SJ. Aquatic therapy for a child with type III spinal muscular atrophy: a case report. Physical & Occupational Therapy in Pediatrics 2010;30(4):313‐24. [PUBMED: 20868338] - PubMed
Vry 2014 {published data only}
-
- Vry J, Schubert IJ, Semler O, Haug V, Schonau E, Kirschner J. Whole‐body vibration training in children with Duchenne muscular dystrophy and spinal muscular atrophy. European Journal of Paediatric Neurology 2014;18(2):140‐9. [PUBMED: 24157400] - PubMed
Additional references
Abresch 2012
-
- Abresch RT, Carter GT, Han JJ, McDonald CM. Exercise in neuromuscular diseases. Physical Medicine and Rehabilitation Clinics North America 2012;23(3):653‐73. [PUBMED: 22938880] - PubMed
ACSM 2010
-
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 8th Edition. Philadelphia: Lippincott Williams & Wilkins, 2010.
Bartels 2015
-
- Bartels B, Takken T, Blank AC, Moorsel H, Pol WL, Groot JF. Cardiopulmonary exercise testing in children and adolescents with dystrophinopathies: a pilot study. Pediatric Physical Therapy 2015;27(3):227‐34. [PUBMED: 26102164] - PubMed
Biondi 2008
Caspersen 1985
Dal Bello‐Haas 2013
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Deymeer 2008
-
- Deymeer F, Serdaroglu P, Parman Y, Poda M. Natural history of SMA IIIb: Muscle strength decreases in a predictable sequence and magnitude. Neurology 2008;71(9):644‐9. [MEDLINE: ] - PubMed
Dunaway 2014
-
- Dunaway S, Montes J, Garber CE, Carr B, Kramer SS, Kamil‐Rosenberg S, et al. Performance of the timed "up & go" test in spinal muscular atrophy. Muscle & Nerve 2014;50(2):273‐7. [PUBMED: 24375426] - PubMed
Dunaway 2016
-
- Dunaway Young S, Montes J, Kramer SS, Marra J, Salazar R, Cruz R, et al. Six‐minute walk test is reliable and valid in spinal muscular atrophy. Muscle & Nerve 2016;54(5):836‐42. [PUBMED: 27015431] - PubMed
Ganley 2011
-
- Ganley KJ, Paterno MV, Miles C, Stout J, Brawner L, Girolami G, et al. Health‐related fitness in children and adolescents. Pediatric Physical Therapy 2011;23(3):208‐20. [PUBMED: 21829112] - PubMed
Garber 2011
-
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Medicine & Science in Sports & Exercise 2011;43(7):1334‐59. [PUBMED: 21694556] - PubMed
GRADEpro GDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 13 September 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Grondard 2005
Higgins 2011
-
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Iannaccone 2009
-
- Iannaccone ST, Hynan LS, Morton A, Buchanan R, Limbers CA, Varni JW. The PedsQL in pediatric patients with spinal muscular atrophy: feasibility, reliability, and validity of the Pediatric Quality of Life Inventory Generic Core Scales and Neuromuscular Module. Neuromuscular Disorders 2009;19(12):805‐12. [PUBMED: 19846309] - PMC - PubMed
Kaufmann 2012
Kruitwagen‐Van Reenen 2016
-
- Kruitwagen‐Van Reenen ET, Wadman RI, Visser‐Meily JM, Berg LH, Schröder C, Pol WL. Correlates of health related quality of life in adult patients with spinal muscular atrophy. Muscle & Nerve 2016;54(5):850‐5. [PUBMED: 27074445] - PubMed
Lefebvre 1995
-
- Lefebvre S, Bürglen L, Reboullet S, Clermont O, Burlet P, Viollet L, et al. Identification and characterization of a spinal muscular atrophy‐determining gene. Cell 1995;80(1):155‐65. [PUBMED: 7813012] - PubMed
Lunn 2008
-
- Lunn MR, Wang CH. Spinal muscular atrophy. Lancet 2008;371(9630):2120‐33. [PUBMED: 18572081] - PubMed
Mazzone 2013
-
- Mazzone E, Bianco F, Main M, Hauwe M, Ash M, Vries R, et al. Six minute walk test in type III spinal muscular atrophy: a 12 month longitudinal study. Neuromuscular Disorders 2013;23(8):624‐8. [PUBMED: 23809874] - PubMed
Mercuri 2012
-
- Mercuri E, Bertini E, Iannaccone ST. Childhood spinal muscular atrophy: controversies and challenges. Lancet Neurology 2012;11(5):443‐52. [PUBMED: 22516079] - PubMed
Mercuri 2018
-
- Mercuri E, Darras BT, Chiriboga CA, Day JW, Campbell C, Connolly AM. Nusinersen versus sham control in later‐onset spinal muscular atrophy. New England Journal of Medicine 2018;378(7):625‐35. [PUBMED: 29443664] - PubMed
Mercuri 2018b
-
- Mercuri E, Finkel RS, Muntoni F, Wirth B, Montes J, Main M, et al. SMA Care Group. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscular Disorders 2018;28(2):103‐15. [PUBMED: 29290580] - PubMed
Montes 2010
Montes 2014
-
- Montes J, Garber CE, Kramer SS, Montgomery MJ, Dunaway S, Kamil‐Rosenberg S, et al. A randomized, controlled clinical trial of exercise in patients with spinal muscular atrophy: methods and baseline characteristics. Journal of Neuromuscular Diseases 2014;1(2):151‐61. [PUBMED: 27858768] - PubMed
Myers 2002
-
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. New England Journal of Medicine 2002;346(11):793‐801. [PUBMED: 11893790] - PubMed
NCT01166022
-
- NCT01166022. Clinical trial of exercise in patients with spinal muscular atrophy (SMA) [Randomized, controlled clinical trial of exercise in patients with spinal muscular atrophy (SMA)]. clinicaltrials.gov/show/NCT01166022 (first received 20 July 2010).
O'Hagen 2007
-
- O'Hagen JM, Glanzman AM, McDermott MP, Ryan PA, Flickinger J, Quigley J, et al. An expanded version of the Hammersmith Functional Motor Scale for SMA II and III patients. Neuromuscular Disorders : NMD 2007;17(9‐10):693‐7. [PUBMED: 17658255] - PubMed
Pedrosa 2015
Piepers 2008
-
- Piepers S, Berg LH, Brugman F, Scheffer H, Ruiterkamp‐Versteeg M, Engelen BG, et al. A natural history study of late onset spinal muscular atrophy types 3b and 4. Journal of Neurology 2008;255(9):1400‐4. [PUBMED: 18575920] - PubMed
Pollock 1998
-
- Pollock ML, Gaesser GA, Butcher JD, Després J‐P, Dishman RK, Franklin BA, et al. American College of Sports Medicine Position Stand: the recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Medicine & Science in Sports & Exercise 1998;30(6):975‐91. [PUBMED: 9624661] - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robertson 2005
-
- Robertson RJ, Goss FL, Andreacci JL, Dube JJ, Rutkowski JJ, Frazee KM, et al. Validation of the Children's OMNI‐Resistance Exercise Scale of perceived exertion. Medicine and Science in Sports and Exercise 2005;37(5):819‐26. [PUBMED: 15870636] - PubMed
Rudnik‐Schöneborn 2001
-
- Rudnik‐Schöneborn S, Hausmanowa‐Petrusewicz I, Borkowska J, Zerres K. The predictive value of achieved motor milestones assessed in 441 patients with infantile spinal muscular atrophy types II and III. European Neurology 2001;45(3):174‐81. [PUBMED: 11306862] - PubMed
Russman 1996
-
- Russman BS, Buncher CR, White M, Samaha FJ, Iannaccone ST. Function changes in spinal muscular atrophy II and III. Neurology 1996;47(4):973‐6. [PUBMED: 8857729] - PubMed
Saunders 2004
-
- Saunders PU, Pyne DB, Telford RD, Hawley JA. Factors affecting running economy in trained distance runners. Sports Medicine 2004;34(7):465‐85. [PUBMED: 15233599] - PubMed
Varni 2004
-
- Varni JW, Burwinkle TM, Szer IS. The PedsQL Multidimensional Fatigue Scale in pediatric rheumatology: reliability and validity. Journal of Rheumatology 2004;31(12):2494‐500. [PUBMED: 15570657] - PubMed
Verhaart 2017
Voet 2013
-
- Voet NBM, Kooi EL, Riphagen II, Lindeman E, Engelen BGM, Geurts ACH. Strength training and aerobic exercise training for muscle disease. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD003907.pub4] - DOI
Vuillerot 2013
-
- Vuillerot C, Payan C, Iwaz J, Ecochard R, Bérard C, MFM Spinal Muscular Atrophy Study Group. Responsiveness of the motor function measure in patients with spinal muscular atrophy. Archives of Physical Medicine and Rehabilitation 2013;94(8):1555‐61. - PubMed
Wadman 2017
-
- Wadman RI, Stam M, Gijzen M, Lemmink HH, Snoeck IN, Wijngaarde CA, et al. Association of motor milestones, SMN2 copy and outcome in spinal muscular atrophy types 0‐4. Journal of Neurology, Neurosurgery, and Psychiatry 2017; Vol. 88, issue 4:365‐7. [PUBMED: 28108522] - PubMed
Wadman 2018
-
- Wadman RI, Wijngaarde CA, Stam M, Bartels B, Otto LAM, Lemmink HH, et al. Muscle strength and motor function throughout life in a cross‐sectional cohort of 180 patients with spinal muscular atrophy types 1c‐4. European Journal of Neurology 2018;25(3):512‐8. [PUBMED: 29194869] - PubMed
Werlauff 2012
-
- Werlauff U, Vissing J, Steffensen BF. Change in muscle strength over time in spinal muscular atrophy types II and III. A long‐term follow‐up study. Neuromuscular Disorders 2012;22(12):1069‐74. [PUBMED: 23127961] - PubMed
Werlauff 2014
-
- Werlauff U, Hojberg A, Firla‐Holme R, Steffensen BF, Vissing J. Fatigue in patients with spinal muscular atrophy type II and congenital myopathies: evaluation of the fatigue severity scale. Quality of Life Research 2014;23(5):1479‐88. [PUBMED: 24214178] - PubMed
Zerres 1997
-
- Zerres K, Rudnik‐Schöneborn S, Forrest E, Lusakowska A, Borkowska J, Hausmanowa‐Petrusewicz I. A collaborative study on the natural history of childhood and juvenile onset proximal spinal muscular atrophy (type II and III SMA): 569 patients. Journal of the Neurological Sciences 1997;146(1):67‐72. [PUBMED: 9077498] - PubMed
References to other published versions of this review
Bartels 2016
-
- Bartels B, Montes J, Pol WL, Groot JF. Skeletal muscle training for spinal muscular atrophy type 3. Cochrane Database of Systematic Reviews 2016, Issue 3. [DOI: 10.1002/14651858.CD012120] - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

