Long-term survival of pig-to-rhesus macaque renal xenografts is dependent on CD4 T cell depletion
- PMID: 30821922
- PMCID: PMC6658347
- DOI: 10.1111/ajt.15329
Long-term survival of pig-to-rhesus macaque renal xenografts is dependent on CD4 T cell depletion
Abstract
The shortage of available organs remains the greatest barrier to expanding access to transplant. Despite advances in genetic editing and immunosuppression, survival in experimental models of kidney xenotransplant has generally been limited to <100 days. We found that pretransplant selection of recipients with low titers of anti-pig antibodies significantly improved survival in a pig-to-rhesus macaque kidney transplant model (6 days vs median survival time 235 days). Immunosuppression included transient pan-T cell depletion and an anti-CD154-based maintenance regimen. Selective depletion of CD4+ T cells but not CD8+ T cells resulted in long-term survival (median survival time >400 days vs 6 days). These studies suggested that CD4+ T cells may have a more prominent role in xenograft rejection compared with CD8+ T cells. Although animals that received selective depletion of CD8+ T cells showed signs of early cellular rejection (marked CD4+ infiltrates), animals receiving selective CD4+ depletion exhibited normal biopsy results until late, when signs of chronic antibody rejection were present. In vitro study results suggested that rhesus CD4+ T cells required the presence of SLA class II to mount an effective proliferative response. The combination of low pretransplant anti-pig antibody and CD4 depletion resulted in consistent, long-term xenograft survival.
Keywords: animal models: nonhuman primate; basic (laboratory) research/science; costimulation; immunosuppressant - fusion proteins and monoclonal antibodies: costimulation molecule specific; immunosuppression/immune modulation; kidney transplantation/nephrology; translational research/science; xenoantibody; xenoantigen; xenotransplantation.
© 2019 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures

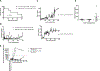


 ) or only αCD8 depletion (
) or only αCD8 depletion ( ) (D) Urine protein measurements remained low post-transplant in long-term survivors until late. (E) Of the long-term survivors treated with αCD4 depletion and anti-CD154, none went on to develop anti-pig antibodies. (F) Representative histology from long-term survivors receiving only αCD4 depletion demonstrated little cellular infiltrate and no glomerulopathy at post-transplant day 200.
) (D) Urine protein measurements remained low post-transplant in long-term survivors until late. (E) Of the long-term survivors treated with αCD4 depletion and anti-CD154, none went on to develop anti-pig antibodies. (F) Representative histology from long-term survivors receiving only αCD4 depletion demonstrated little cellular infiltrate and no glomerulopathy at post-transplant day 200.

References
-
- Organ Procurement and Transplantation Network [Internet] [cited 2017. Jan 1]. Available from: https://optn.transplant.hrsa.gov/
-
- Wolfe R, Roys EC, Merion RM. Trends in Organ Donation and Transplantation in the United States, 1999–2008. Am J Transplant 2010;10(4 Pt 2):961–72. - PubMed
-
- Lai L, Kolber-Simonds D, Park K, Cheong H, Greenstein J, Im G, et al. Production of alpha −1,3-Galactosyltransferase Knockout Pigs by Nuclear Transfer Cloning. Science 2002;295(5557):1089–92. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

