Conserved HA-peptide NG34 formulated in pCMV-CTLA4-Ig reduces viral shedding in pigs after a heterosubtypic influenza virus SwH3N2 challenge
- PMID: 30822308
- PMCID: PMC6396909
- DOI: 10.1371/journal.pone.0212431
Conserved HA-peptide NG34 formulated in pCMV-CTLA4-Ig reduces viral shedding in pigs after a heterosubtypic influenza virus SwH3N2 challenge
Abstract
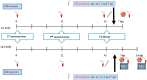
Swine influenza viruses (SIVs), the causal agents of swine influenza, are not only important to control due to the economic losses in the swine industry, but also can be pandemic pathogens. Vaccination is one of the most relevant strategies to control and prevent influenza infection. Current human vaccines against influenza induce strain-specific immunity and annual update is required due to the virus antigenic shift phenomena. Previously, our group has reported the use of conserved hemagglutinin peptides (HA-peptides) derived from H1-influenza virus as a potential multivalent vaccine candidate. Immunization of swine with these HA-peptides elicited antibodies that recognized and neutralized heterologous influenza viruses in vitro and demonstrated strong hemagglutination-inhibiting activity. In the present work, we cloned one HA-peptide (named NG34) into a plasmid fused with cytotoxic T lymphocyte-associated antigen (CTLA4) which is a molecule that modifies T cell activation and with an adjuvant activity interfering with the adaptive immune response. The resulting plasmid, named pCMV-CTLA4-Ig-NG34, was administered twice to animals employing a needle-free delivery approach. Two studies were carried out to test the efficacy of pCMV-CTLA4-Ig-NG34 as a potential swine influenza vaccine, one in seronegative and another in seropositive pigs against SIV. The second one was aimed to evaluate whether pCMV-CTLA4-Ig-NG34 vaccination would overcome maternally derived antibodies (MDA). After immunization, all animals were intranasally challenged with an H3N2 influenza strain. A complete elimination or significant reduction in the viral shedding was observed within the first week after the challenge in the vaccinated animals from both studies. In addition, no challenged heterologous virus load was detected in the airways of vaccinated pigs. Overall, it is suggested that the pCMV-CTLA4-Ig-NG34 vaccine formulation could potentially be used as a multivalent vaccine against influenza viruses.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
DNA vaccine based on conserved HA-peptides induces strong immune response and rapidly clears influenza virus infection from vaccinated pigs.PLoS One. 2019 Sep 25;14(9):e0222201. doi: 10.1371/journal.pone.0222201. eCollection 2019. PLoS One. 2019. PMID: 31553755 Free PMC article.
-
Protection of guinea pigs by vaccination with a recombinant swinepox virus co-expressing HA1 genes of swine H1N1 and H3N2 influenza viruses.Arch Virol. 2013 Mar;158(3):629-37. doi: 10.1007/s00705-012-1539-9. Epub 2012 Nov 8. Arch Virol. 2013. PMID: 23135159
-
A Bivalent Heterologous DNA Virus-Like-Particle Prime-Boost Vaccine Elicits Broad Protection against both Group 1 and 2 Influenza A Viruses.J Virol. 2017 Apr 13;91(9):e02052-16. doi: 10.1128/JVI.02052-16. Print 2017 May 1. J Virol. 2017. PMID: 28179535 Free PMC article.
-
Vaccine development for protecting swine against influenza virus.Anim Health Res Rev. 2012 Dec;13(2):181-95. doi: 10.1017/S1466252312000175. Anim Health Res Rev. 2012. PMID: 23253165 Review.
-
Influenza A Virus in Swine: Epidemiology, Challenges and Vaccination Strategies.Front Vet Sci. 2020 Sep 22;7:647. doi: 10.3389/fvets.2020.00647. eCollection 2020. Front Vet Sci. 2020. PMID: 33195504 Free PMC article. Review.
Cited by
-
Skin-Based Vaccination: A Systematic Mapping Review of the Types of Vaccines and Methods Used and Immunity and Protection Elicited in Pigs.Vaccines (Basel). 2023 Feb 16;11(2):450. doi: 10.3390/vaccines11020450. Vaccines (Basel). 2023. PMID: 36851328 Free PMC article. Review.
-
Swine influenza A virus: challenges and novel vaccine strategies.Front Cell Infect Microbiol. 2024 Apr 3;14:1336013. doi: 10.3389/fcimb.2024.1336013. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38633745 Free PMC article. Review.
-
Targeting Hemagglutinin: Approaches for Broad Protection against the Influenza A Virus.Viruses. 2019 Apr 30;11(5):405. doi: 10.3390/v11050405. Viruses. 2019. PMID: 31052339 Free PMC article. Review.
-
Influenza NG-34 T cell conserved epitope adjuvanted with CAF01 as a possible influenza vaccine candidate.Vet Res. 2020 Apr 20;51(1):57. doi: 10.1186/s13567-020-00770-4. Vet Res. 2020. PMID: 32312317 Free PMC article.
-
In Silico Exploration of CD200 as a Therapeutic Target for COVID-19.Microorganisms. 2024 Jun 12;12(6):1185. doi: 10.3390/microorganisms12061185. Microorganisms. 2024. PMID: 38930566 Free PMC article.
References
-
- Zimmerman J, Karriker L, Ramírez A, Schwartz A, Stevenson G. Diseases of swine, 10th Edition 2012.
-
- Kothalawala H, Toussaint MJM, Gruys E. An overview of swine influenza Vet Q. 2006;28: 46–53. http://www.ncbi.nlm.nih.gov/pubmed/16841566 - PubMed
-
- Loeffen WL, Kamp EM, Stockhofe-Zurwieden N, van Nieuwstadt AP, Bongers JH, Hunneman WA, et al. Survey of infectious agents involved in acute respiratory disease in finishing pigs. Vet Rec. 1999;145: 123–9. Available: http://www.ncbi.nlm.nih.gov/pubmed/10466829 - PubMed

