A computational model of feedback-mediated hematopoietic stem cell differentiation in vitro
- PMID: 30822334
- PMCID: PMC6396932
- DOI: 10.1371/journal.pone.0212502
A computational model of feedback-mediated hematopoietic stem cell differentiation in vitro
Abstract
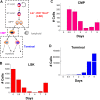
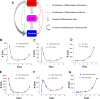
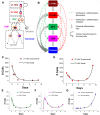
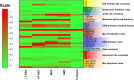
Hematopoietic stem cells (HSCs) play an important physiological role as regulators of all blood and immune cell populations, and are of clinical importance for bone marrow transplants. Regulating HSC biology in vitro for clinical applications requires improved understanding of biological inducers of HSC lineage specification. A significant challenge for controlled HSC expansion and differentiation is the complex network of molecular crosstalk between multiple bone marrow niche components influencing HSC biology. We describe a biology-driven computational approach to model cell kinetics in vitro to gain new insight regarding culture conditions and intercellular signaling networks. We further investigate the balance between self-renewal and differentiation that drives early and late hematopoietic progenitor populations. We demonstrate that changing the feedback driven by cell-secreted biomolecules alters lineage specification in early progenitor populations. Using a first order deterministic model, we are able to predict the impact of media change frequency on cell kinetics, as well as distinctions between primitive long-term HSCs and differentiated myeloid progenitors. Integrating the computational model and sensitivity analyses we identify critical culture parameters for regulating HSC proliferation and myeloid lineage specification. Our analysis suggests that accurately modeling the kinetics of hematopoietic sub-populations in vitro requires direct contributions from early progenitor differentiation along with the more traditionally considered intermediary oligopotent progenitors. While consistent with recent in vivo results, this work suggests the need to revise our perspective on HSC lineage engineering in vitro for expansion of discrete hematopoietic populations.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

