High-Pressure Response of Amyloid Folds
- PMID: 30823361
- PMCID: PMC6466028
- DOI: 10.3390/v11030202
High-Pressure Response of Amyloid Folds
Abstract
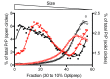
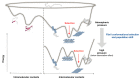
The abnormal protein aggregates in progressive neurodegenerative disorders, such as Alzheimer's, Parkinson's and prion diseases, adopt a generic structural form called amyloid fibrils. The precise amyloid fold can differ between patients and these differences are related to distinct neuropathological phenotypes of the diseases. A key focus in current research is the molecular mechanism governing such structural diversity, known as amyloid polymorphism. In this review, we focus on our recent work on recombinant prion protein (recPrP) and the use of pressure as a variable for perturbing protein structure. We suggest that the amyloid polymorphism is based on volumetric features. Accordingly, pressure is the thermodynamic parameter that fits best to exploit volume differences within the states of a chemical reaction, since it shifts the equilibrium constant to the state that has the smaller volume. In this context, there are analogies with the process of correct protein folding, the high pressure-induced effects of which have been studied for more than a century and which provides a valuable source of inspiration. We present a short overview of this background and review our recent results regarding the folding, misfolding, and aggregation-disaggregation of recPrP under pressure. We present preliminary experiments aimed at identifying how prion protein fibril diversity is related to the quaternary structure by using pressure and varying protein sequences. Finally, we consider outstanding questions and testable mechanistic hypotheses regarding the multiplicity of states in the amyloid fold.
Keywords: amyloid; high pressure; polymorphism; prion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

