Autophagy in Zika Virus Infection: A Possible Therapeutic Target to Counteract Viral Replication
- PMID: 30823365
- PMCID: PMC6429311
- DOI: 10.3390/ijms20051048
Autophagy in Zika Virus Infection: A Possible Therapeutic Target to Counteract Viral Replication
Abstract
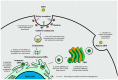
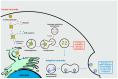
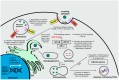
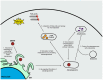
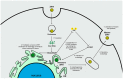
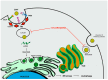
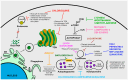
Zika virus (ZIKV) still constitutes a public health concern, however, no vaccines or therapies are currently approved for treatment. A fundamental process involved in ZIKV infection is autophagy, a cellular catabolic pathway delivering cytoplasmic cargo to the lysosome for degradation-considered as a primordial form of innate immunity against invading microorganisms. ZIKV is thought to inhibit the Akt-mTOR signaling pathway, which causes aberrant activation of autophagy promoting viral replication and propagation. It is therefore appealing to study the role of autophagic molecular effectors during viral infection to identify potential targets for anti-ZIKV therapeutic intervention.
Keywords: Akt-mTOR; Zika virus; autophagy; innate immunity; therapeutic targets.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Mechanistic Target of Rapamycin Signaling Activation Antagonizes Autophagy To Facilitate Zika Virus Replication.J Virol. 2020 Oct 27;94(22):e01575-20. doi: 10.1128/JVI.01575-20. Print 2020 Oct 27. J Virol. 2020. PMID: 32878890 Free PMC article.
-
Zika Virus Induces Autophagy in Human Umbilical Vein Endothelial Cells.Viruses. 2018 May 15;10(5):259. doi: 10.3390/v10050259. Viruses. 2018. PMID: 29762492 Free PMC article.
-
Favipiravir and Ribavirin Inhibit Replication of Asian and African Strains of Zika Virus in Different Cell Models.Viruses. 2018 Feb 9;10(2):72. doi: 10.3390/v10020072. Viruses. 2018. PMID: 29425176 Free PMC article.
-
ZIKA virus entry mechanisms in human cells.Infect Genet Evol. 2019 Apr;69:22-29. doi: 10.1016/j.meegid.2019.01.018. Epub 2019 Jan 15. Infect Genet Evol. 2019. PMID: 30658214 Review.
-
Role of autophagy in Zika virus infection and pathogenesis.Virus Res. 2018 Aug 2;254:34-40. doi: 10.1016/j.virusres.2017.09.006. Epub 2017 Sep 9. Virus Res. 2018. PMID: 28899653 Free PMC article. Review.
Cited by
-
Cell deaths: Involvement in the pathogenesis and intervention therapy of COVID-19.Signal Transduct Target Ther. 2022 Jun 13;7(1):186. doi: 10.1038/s41392-022-01043-6. Signal Transduct Target Ther. 2022. PMID: 35697684 Free PMC article. Review.
-
Catch Me if You Can: the Crosstalk of Zika Virus and the Restriction Factor Tetherin.J Virol. 2022 Feb 23;96(4):e0211721. doi: 10.1128/jvi.02117-21. Epub 2021 Dec 22. J Virol. 2022. PMID: 34935441 Free PMC article.
-
Novel Therapeutic Nutrients Molecules That Protect against Zika Virus Infection with a Special Note on Palmitoleate.Nutrients. 2022 Dec 27;15(1):124. doi: 10.3390/nu15010124. Nutrients. 2022. PMID: 36615782 Free PMC article. Review.
-
Zika virus is transmitted in neural progenitor cells via cell-to-cell spread and infection is inhibited by the autophagy inducer trehalose.J Virol. 2021 Mar 1;95(5):e02024-20. doi: 10.1128/JVI.02024-20. Epub 2020 Dec 16. J Virol. 2021. PMID: 33328307 Free PMC article.
-
Transcriptional signatures of Zika virus infection in astrocytes.J Neurovirol. 2021 Feb;27(1):116-125. doi: 10.1007/s13365-020-00931-3. Epub 2021 Jan 6. J Neurovirol. 2021. PMID: 33405202 Free PMC article.
References
-
- Secretaria de Vigilância em Saúde, Ministério da Saúde Monitoramento integrado de alterações no crescimento e desenvolvimento relacionadas à infecção pelo vírus Zika e outras etiologias infecciosas, até a Semana Epidemiológica 40 de 2018. Boletim Epidemiológico. 2018;49:1–8.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

