Natural Products Containing 'Rare' Organophosphorus Functional Groups
- PMID: 30823503
- PMCID: PMC6429109
- DOI: 10.3390/molecules24050866
Natural Products Containing 'Rare' Organophosphorus Functional Groups
Abstract
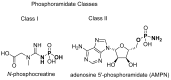
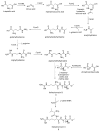
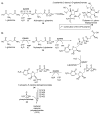
Phosphorous-containing molecules are essential constituents of all living cells. While the phosphate functional group is very common in small molecule natural products, nucleic acids, and as chemical modification in protein and peptides, phosphorous can form P⁻N (phosphoramidate), P⁻S (phosphorothioate), and P⁻C (e.g., phosphonate and phosphinate) linkages. While rare, these moieties play critical roles in many processes and in all forms of life. In this review we thoroughly categorize P⁻N, P⁻S, and P⁻C natural organophosphorus compounds. Information on biological source, biological activity, and biosynthesis is included, if known. This review also summarizes the role of phosphorylation on unusual amino acids in proteins (N- and S-phosphorylation) and reviews the natural phosphorothioate (P⁻S) and phosphoramidate (P⁻N) modifications of DNA and nucleotides with an emphasis on their role in the metabolism of the cell. We challenge the commonly held notion that nonphosphate organophosphorus functional groups are an oddity of biochemistry, with no central role in the metabolism of the cell. We postulate that the extent of utilization of some phosphorus groups by life, especially those containing P⁻N bonds, is likely severely underestimated and has been largely overlooked, mainly due to the technological limitations in their detection and analysis.
Keywords: N-phosphorylation; P–C bond; P–N bond; P–S bond; S-phosphorylation; phosphinate; phosphine; phosphonate; phosphoramidate; phosphorothioate.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

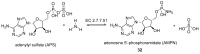
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

